A semiconductor is a substance that, by its specific electrical conductivity is located between the conductor and the dielectric (they have a narrow band gap), and differs from the conductor in the strong dependence of conductivity on external influences and impurity concentration.
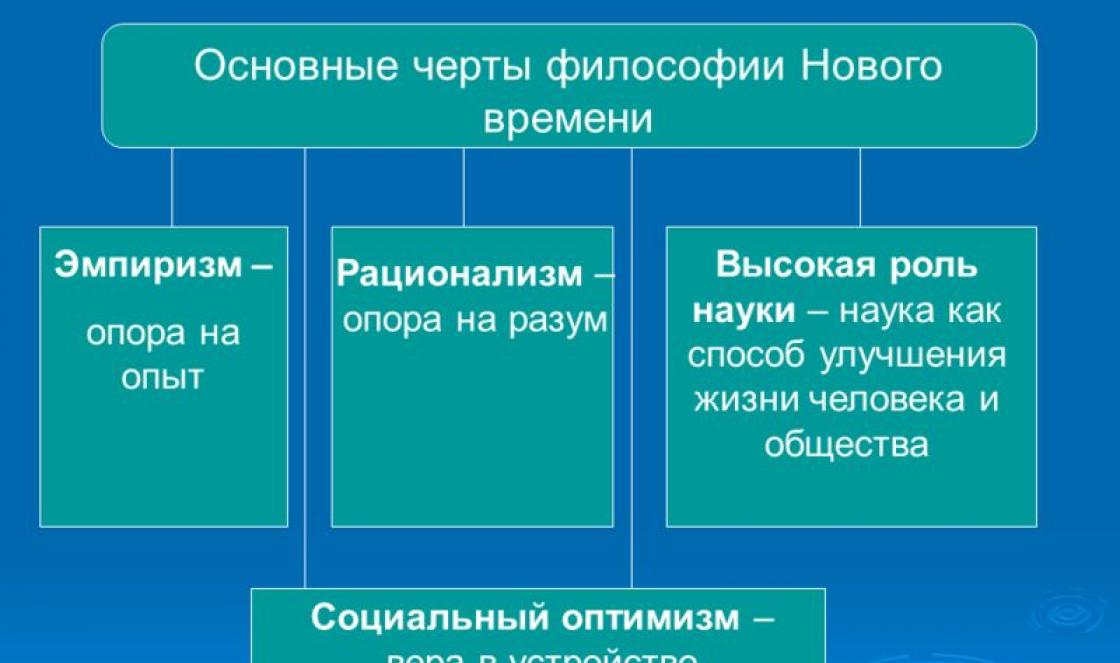
1.1 Band theory of semiconductors
If the electron of an atom in the crystal lattice remains associated with the nucleus, then it is in the valence band, if it is torn off from the nucleus, then in the conduction band. Between these zones there is a band gap. An electron cannot possess such energy (Fig. 1-1).
Fig.1-1 Energy zones
Semiconducting properties can be possessed by both simple substances, for example, diamond C, tellurium Te, selenium Se (red), gray tin - Sn, and chemical organic and inorganic compounds: gallium arsenide GaAs, indium antimonide InSb, indium phosphide InP, silicon carbide SiC , benzene, naphthalene, naphthacene, etc. Typical representatives of semiconductors are elements of the fourth group periodic system: germanium Ge and silicon Si.
Semiconductor atoms in a crystal lattice are interconnected by pair-electron (covalent) bonds. These bonds are fragile, easily broken when heated, illuminated, electrified.
The bonds are fragile, easily broken when heated, illuminated, electrified (Fig. 1-2).

Fig.1-2 Semiconductor crystal lattice
When an electron is removed, a hole remains, which has a positive charge equal to the charge of the electron. In a pure semiconductor, the number of electrons and holes is the same n p =n n =n i .
The number of charge carriers n i =Ae ΔE /kT - depends on temperature and band gap.
Each covalent bond is formed by a pair of electrons, made up of one electron from the first, and one from the second atoms. In a chemically pure semiconductor, all covalent bonds are filled and at a temperature of absolute zero, unlike metals, semiconductors have no free charge carriers. With increasing temperature environment part of the electrons is excited and, breaking covalent bond, passes into the conduction band, creating the intrinsic electronic conductivity of the semiconductor. At the same time, an unfilled covalent bond, a hole, appears in the semiconductor. Such a bond can be restored at the expense of an electron of a neighboring atom, i.e. destruction of an adjacent covalent bond. Repeated repetition of such situations creates the appearance of a hole moving through the volume of the crystal, which, having a positive charge, creates its own hole conductivity of the semiconductor. The process of generation of electron-hole pairs can occur not only under the influence of heat, but also due to any processes capable of imparting to an electron the amount of energy sufficient to break the covalent bond. The generation process is always accompanied by the reverse process - recombination, that is, the connection of an electron with a hole to form a neutral atom. As a result, under constant external conditions, an equilibrium occurs in the semiconductor, at which the number of generated pairs of charge carriers is equal to the number of recombining ones.
1.2 Impurity semiconductors
In a pure semiconductor, the formation of a pair requires a significant amount of energy and its conductivity at room temperature is very small.
It is possible to significantly increase the conductivity by doping the semiconductor with trivalent or pentavalent impurities. In a pentavalent impurity (antimony Sb, phosphorus P, arsenic As), one electron does not participate in covalent bonds and easily passes into the free zone when the energy is given to it much less than is necessary to break the covalent bond. As a result, the impurity atom, having donated an electron, becomes a stable immobile positive ion. Such impurities are called donor impurities. and the semiconductors doped with them are n-type semiconductors. The conductivity of an impurity semiconductor is commonly called impurity conductivity. The majority charge carriers in an n-type semiconductor are electrons, while the minority carriers are holes.
A trivalent impurity atom, on the contrary, tends to take an electron from the nearest semiconductor atom to fill the fourth covalent bond. In this case, a stable negative ion and a hole are formed. A semiconductor with such impurities is called a p-type semiconductor, the impurities themselves (aluminum Al, boron B, indium In.) are acceptor. In a p-type semiconductor, the majority charge carriers are holes, and the minority carriers are electrons.
In impurity semiconductors at room temperature, almost all impurity atoms are in an excited state, and the number of majority carriers created by them far exceeds the number of minority carriers that arise by ordinary thermal generation of electron-hole pairs. As a result, the impurity conductivity is much higher than the intrinsic conductivity of the semiconductor, depends to a much lesser extent on external factors, and is determined mainly by the concentration of the dopant.
1.3 Semiconductor diode
The basis of all semiconductor devices is the electron-hole transition ( p-n junction). It is formed at the boundary of two semiconductors with different types of conductivity (chapter 1.2). Since the concentration of charge carriers in area r-n transition is sharply inhomogeneous, according to the laws of diffusion, the main carriers (holes in the "p" region and electrons in the "n" region) will diffuse into adjacent regions, creating a diffusion current.
Minor charge carriers (holes in the n-region and electrons in the p-region) will begin to drift in the emerging electric field, creating a drift current directed towards the diffusion current. As a result, dynamic equilibrium sets in, the total junction current will be zero, and a contact potential difference will be established at the junction, which is 0.3-0.4 V for germanium junctions and 0.7-1.0 V for silicon junctions. If you connect an emf source to the junction with a positive pole to the p region, and a negative pole to the n region, then the resulting potential difference at the junction will decrease. The transition will open and begin to conduct current due to an increase in the diffusion of the main charge carriers from the n-region to the p-region. In this case, the drift current through the junction will decrease. Such an inclusion of a transition is usually called an inclusion in the forward direction (a forward-biased transition).

Fig 1-3 Direct connection of p-n junction
The application of voltage in the opposite direction (plus to n, and minus to the p-region) will lead to an increase in the potential difference at the junction, and hence to a decrease in the diffusion current and an increase in the drift current. Since the drift current is created by minority charge carriers, which are much smaller in a semiconductor than the main ones, the total current through the junction will be very small. This transition state is called closed.

Fig.1-4 Reverse inclusion of p-n junction.
In the absence of external electric field, the diffusion current is equal to the conduction current.
I transition \u003d I diff -I wire \u003d 0.
1. If the applied external field enhances the transition field (+ to the n layer), then I diff will decrease, I prov will increase.
I transition \u003d -I 0 (reverse current).
2. If we weaken the transition field (+ to the p layer), then I diff increases, I prov decreases. I transition >> I 0, I transition \u003d I ave.
Therefore, the p-n junction is called a semiconductor diode.
Its designation in schemes + p ![]() -n
-n
Semiconductor devices, consisting of one p-n junction and designed to rectify alternating current, are called rectifier diodes. In such diodes, the main property of the transition is used - the ability to conduct current well in only one direction.
Characteristics of a semiconductor diode

Figure 1-5 Diode Forward and Reverse
The main parameters of the rectifier diode are: the maximum value of the rectified current Irec, the forward voltage drop at the junction at the maximum rectified current Upr, the maximum allowable reverse voltage Uo6p, the magnitude of the reverse current Io at Uo6p. Usually Ivyp = 10 mA - 10 A; Unp = 0.2 - 1.5 V; Uo6p = 10 V - 1kV Io = 1 μA - 100 μA.
If the reverse voltage in a rectifier diode exceeds the breakdown voltage Uprob (usually Uo6p = 0.8Uprob), the current will increase sharply and the diode will fail, which is explained by an increase in the number of carriers in the junction region under the action of impact ionization in a strong electric field and subsequent enhanced thermal generation of the heated junction .
Marking (designation) of diodes
Letters and numbers are used in the designation of the diode:
G (or 1) - germanium diode; K (or 2) - silicon diode.

Figure 1-6 Appearance semiconductor diodes
1.4 Zener diode
By increasing the concentration of impurities (Chapter 1.2), in silicon diodes, it is possible to achieve the reversibility of the process of electrical breakdown. In this case, a section is formed on the reverse branch of the I–V characteristic (chapter 1.3), where large changes in current through the junction cause small changes in voltage (Fig. 1-7). Diodes with such a CVC are called zener diodes, or reference diodes, since they are used to stabilize the voltage.

Figure 1-7 Zener diode volt-ampere characteristic
The main parameters of the zener diodes are: Imin, Imax, respectively, the minimum and maximum stabilization currents that determine the working section of the CVC. Typically Imin is between 3mA and 100mA and Imax is between 10mA and 3A.
Ustab.nom - rated stabilization voltage, usually from 1 to 200 V;
Rdin \u003d dU / dI - dynamic resistance, where dI, dU - increments of current and voltage in the working section of the current-voltage characteristic, usually Rdin \u003d 10-100 Ohms.
At the zener diode, the reverse voltage remains almost constant under the condition
I arr max>= I>= I arr, min.

Fig.1-8 Zener diode switching circuit
U non-stab \u003d U stub + I stub R limited
U stub = 3.3 V - 150 V
I stub, min = 2 - 5 mA
I stub, max = 30 - 500 mA
Stabilizing properties are characterized by a stabilization coefficient:
K stub \u003d (ΔU non-stab U stub) / (U non-stab ΔU stub) K stub \u003d 5-10.
To increase the stabilization coefficient, a cascade connection of stabilizing cells is used.

Fig. 1-9 Cascade connection of zener diodes
The disadvantage of a multi-cell stabilizer is the large voltage losses across the limiting resistors. To increase the stabilized voltage, a series connection of zener diodes is used.

Fig.1-10 Series connection of zener diodes
If the zener diodes are turned on in opposite directions, then when an alternating voltage is applied to them, a two-sided limitation of the output voltage occurs (Fig.

Fig.1-11 Zener Diode Back-to-Back Connection
Parallel connection of zener diodes is not applicable. at the moment of switching on, the zener diode with the smallest Ustab always opens and the rest of the zener diodes remain closed.
Fig.1-12 Appearance of zener diodes
1-low power zener diode;
2-power zener diode with heatsink mount
1.5 Transistors
1.5.1 Transistor structure
The transistor is a three-layer semiconductor device with layers of alternating type of conductivity (chapter 1.2). There are pnp and npn type transistors.

Fig.1-13 Transistor structure
emitter– p-semiconductor with big amount impurities.
Base– n-semiconductor with a small amount of impurities. The base layer is very thin, about 1 micron.
Collector– p semiconductor with an average amount of impurities. The emitter-base junction is called the emitter junction, the base-collector junction is called the collector junction.
Most often, the transistor is turned on so that the emitter junction is turned on in the forward direction, and the collector junction is turned on in the opposite direction.
When the transistor is turned on, a large number of holes are injected from the emitter into the base, which propagate in the base by diffusion, reach the collector junction and are drawn in by it, forming a large collector current. I to- ≈I e, but I to-< I э. Поведение транзистора описывается 2-я уравнениями:
I e \u003d I b + I k and I k \u003d αI e + I k0, where α is the current transfer coefficient of the transistor connected according to the common base circuit (OB). α=0.9 – 0.995.

Figure 1-14 Transistor Symbols

Fig.1-15 Appearance of transistors of different power
1.5.2 Transistor circuits
1. Scheme with a common base (OB)
Fig.1-16 OB diagram
The transistor can be used to amplify the signal. If U kb \u003e U eb and R k \u003e R e, then with almost the same currents in the emitter and collector circuits, R k will have a significantly larger voltage drop than R e, that is, the voltage is amplified, and hence the signal power.
2. Circuit with a common emitter (CE):
Fig.1-17 OE diagram
A transistor connected according to the OE circuit amplifies both voltage and current. I e \u003d I k + I b and I k \u003d βI b + (β + 1) I kb0, where β is the current transfer coefficient in a circuit with a common emitter. β=α/(1-α), depends on the thickness of the base and is within β=10 - 200.
3. Scheme with a common collector (OK)
Fig.1-18 OK diagram
In this circuit, U out< U вх, но U вых ≈ U вх то есть усиление по напряжению не происходит, но усиливается ток приблизительно в β раз. Поэтому схема называется эмиттерный повторитель (повторяет напряжение).
1.5.3 Transistor characteristics (OB circuit)

Fig.1-19 1.2 Input and output characteristics
1. Input characteristics: I e \u003d f (U eb) with U kb \u003d const.
2. Output characteristics: I k \u003d f (U kb) with I e \u003d const.
3 Flow characteristics: I to =f(I e) at U kb =const.

Fig 1-19 Flow characteristics
When Uk = 0, the input characteristic is a direct branch of the BAX emitter p-n - junction. With an increase in Ukb, the I–V characteristic shifts to the left, since an increase in the reverse collector current additionally opens the p-n junction and Ie ≠ 0 at Ueb = 0. For Ie = 0, the output characteristic is the opposite branch of the collector junction. If Ie> 0, then Ik> 0 even at Ukb = 0 due to the capture of charge carriers injected by the emitter by the field of the potential barrier of the collector junction. At the same time, with an increase in Ueb, Ie quickly reaches its maximum value, since even at low Ucb, the main part of the injected carriers is captured by the collector.
1.5.4 Physical model of the transistor
When calculating electronic circuits, the real transistor in the circuit is replaced by the model below, which accurately reflects its properties.

Fig.1-20 Physical model of the transistor
R e \u003d 10 - 30 Ohm, R b \u003d 100 - 300 Ohm, R k \u003d 10 4 - 10 5 Ohm
The transistor can be considered as a four-terminal:

Fig.1-21 Transistor as a quadripole
Then it can be described by a system of h parameters:

To determine the h-parameters, we will use the short circuit and open circuit method.
a) Output short circuit. Therefore U 2 =0.
h 11 \u003d Z in - input resistance
h 21 b \u003d α - current gain in a circuit with a common base
h 21 E \u003d β - current gain in a circuit with a common emitter
b) Idling at the input (I 1 \u003d 0), then
U 1 \u003d h 12 U 2, h 12 \u003d U 1 / U 2 - voltage reverse transfer coefficient
I 2 \u003d h 22 U 2, h 22 \u003d I 2 /U 2 \u003d y out - output conductivity.
1.5.5 Field (channel) transistors (PT)
FET - a semiconductor device in which the current through the channel is controlled by an electric field that occurs with the application of voltage between the gate and source. In a PT, unlike a bipolar transistor (Chapter 1.5.1), charge carriers of only one sign (only electrons or only holes) move along a semiconductor channel.
A channel is a region in a transistor whose resistance depends on the potential at the gate. The electrode from which the main charge carriers enter the channel is called the source, and the electrode through which the main charge carriers leave the channel is called the drain. The electrode that regulates the cross section of the channel is called a gate.
FETs are made of silicon and, depending on the electrical conductivity of the source material, are divided into transistors with p and n channels - types.
Field-effect transistor with a gate in in the form of p-n transition
This is a semiconductor device in which the conductivity of the channel can be controlled by applying a voltage to closed district transition. Figure 1-22 shows the structure, switching circuit and symbol of a FET with an n-type channel and a gate in the form of a p-n junction.
In FETs with an n-type channel, the main charge carriers in the channel are electrons that move along the channel from a source with a low potential to a drain with a higher potential, forming a drain current. Ic. A voltage is applied between the gate and the source, blocking the pn junction formed by the n-region of the channel and the p-region of the gate.
Therefore, in a PT with an n-type channel Usi>0, Uzi<0. В ПТ с каналом р-типа Uси<0, Uзи>0.

Figure 1-22 FET
1-drain pin; 2-gate; 3-channel; 4-gate pin; 5-drain pin
Figure 1-23 shows how the channel cross section changes due to the change in the width of the barrier layer when voltages are applied between the transistor electrodes. When a blocking voltage is applied to the p-n junction between the gate and the channel (Fig. 1-23a), uniform layers appear that are depleted in charge carriers and have a high resistivity, which leads to a decrease in the channel width.

Fig.1-23. Overlapping of the PT channel at different voltages on the electrodes
The voltage applied between the drain and the source (Fig. 1-236) causes the appearance of an uneven depletion layer, since the potential difference between the gate and the channel increases in the direction from the source to the drain and the smallest section of the channel is located near the drain.
If you simultaneously apply voltage Usi>0 and Uzi<0 (рис.1-22в), то сечение канала будет определяться действием этих двух напряжений. Минимальное сечение канала определяется их суммой:Uси+|Uзи|.Когда суммарное напряжение достигает напряжения запирания:Uси+|Uзи|=Uзап, обеднённые области смыкаются, ширина канала уменьшается до капилляра и динамическое сопротивление резко возрастает.
The dependence of the drain current Ic on the voltage Usi at a constant gate voltage Uzi determines the output or drain characteristics (Fig. 1-24).

Figure 1-24. Output current-voltage characteristics of an n-channel FET.
In the initial section of the characteristic Usi + |Uzi|< Uзап и ток Iс возрастает с повышением Uси. При повышении напряжения сток-исток до величины Uси =Uзап- |Uзи| происходит смыкание канала, и рост тока Iс прекращается (участок насыщения). Отрицательное напряжение, приложенное к затвору ПТ. смещает момент перекрытия канала в сторону меньших значений напряжения U и тока Iс. Дальнейшее повышение напряжения Uси приводит к пробою р-n перехода между затвором и каналом, что выводит транзистор из строя.
According to the output characteristics of the PT, it is possible to construct the transient characteristic Iс =f(Uzi) . In the saturation region, it practically does not depend on the voltage Us.
The input characteristic of the FET: Ig = f (Ug) is not used, since the transition between the gate and the channel is closed, the gate current is very small and can be neglected.
Insulated Gate Field Effect Transistor
This is a semiconductor device, in which, to further reduce the gate leakage current Iz, a thin layer of dielectric (SiO2) is located between the metal gate and the channel, and there is no p-n junction. Such FETs are called MOSFETs (metal-dielectric-semiconductor structure).

Figure 1-25 Insulated Gate FET
The current-voltage characteristics of an FET with an insulated gate are similar to those of a FET with a p-n junction gate. But an insulated gate allows you to work even at a voltage Uzi>0, when the channel expands and the current Ic increases.
The main parameters of the PT:
1) the slope of the transient characteristic S = dIc / dUzi at Usi = const and
2) differential resistance of the drain (channel) in the saturation section Rc=dUsi/dIс at Uзi = const.
1.6 Other semiconductor devices
1.6.1 Thyristors

Fig.1-26 Thyristor
Thyristor is a four-layer semiconductor device. the chain is very small (plot 1). At a certain voltage U on, an avalanche breakdown begins and the current increases sharply (section 3) - the thyristor turns on.
The output of the control electrode is connected to the middle p (or n) layer At. By applying a small voltage U control to it, you can reduce the turn-on voltage U on.
Figure 1-27 shows the process of turning on thyristors using a control electrode. A thyristor is connected between the source and load R load. Since U pete< U вкл, то тиристор закрыт, тока в нагрузке нет (рис.1). При подаче короткого положительного импульса от блока управления тиристор включается(рис.2) и дальше становится неуправляемым. Выключить его можно только снизив ток до величины I выкл. При работе тиристора в цепи переменного тока это происходит автоматически.

Figure 1-27 Thyristor control circuit
1.6.2 Photoelectronic semiconductor devices
Exists whole line diodes using a variety of phenomena and effects that take place in the p-n junction (chapter 1.3). Thus, a varicap (voltage controlled capacitance) uses the dependence of the capacitance of a reverse-biased junction on the applied voltage. The photodiode is based on the phenomenon of the generation of charge carriers in the transition region and the appearance of photovoltage under the action of light. The LED is based on the property of electron-hole pairs to emit a quantum of light during their recombination, etc.

Fig.1-28 Types of optoelectronic devices

Fig.1-29 Photodiode in the photoEMF generation mode.
At lighting r-p transition, the covalent bonds are broken; the formed minority carriers (Chapter 1.5.1) are drawn in by the transition. In the layers, the number of major carriers increases (holes in the p-layer, electrons in the p-layer), which creates a potential difference between the layers, depending on the illumination of the transition ( fig 1-29).

Fig.1-30
If an EMF source is included in the photodiode circuit in the opposite direction (Fig. 1-30), then the number of carriers increases during illumination and the reverse current increases in proportion to the magnitude of the luminous flux F. The resulting current almost does not depend on the magnitude of the applied voltage (Fig. 1-31).


Fig.1-31 CVC of photodiode Fig.1-32 Turning on the LED
The LED is a radiating p-p junction, the glow of which is caused by the recombination of carriers when the junction is shifted in the forward direction under the action of an applied voltage (Fig. 1-32).
A phototransistor is an ordinary transistor (Chapter 1.5.1), in the case of which a window is made through which the light flux enters the base. When the base of the phototransistor is illuminated, the formed carriers are drawn in by transitions, the base current increases. This causes a much larger change in collector current as the transistor is connected to an EMF source.
An optocoupler is a semiconductor device in which signals are transmitted from the input to the output part of an electronic device using photons, without the use of galvanic, magnetic or other connections.
An optocoupler consists of an LED, the optical radiation of which acts on a light receiver - a photoresistor, photothyristor or phototransistor. Both semiconductor elements are enclosed in a common housing. The outputs from the LED are the input, and the outputs from the light receiver are the output of the optocoupler. The value of the output signal of the optocoupler is controlled by changing the value of the input signal.
1.6.3 Integrated circuits
A microcircuit is a structurally complete microelectronic product that performs a certain function of information conversion, containing a set of electrically interconnected electrical radio elements (transistors, diodes, resistors, capacitors, etc.) manufactured in a single technological cycle.
Microcircuits are manufactured by a group method, replicating simultaneously in one batch from several tens to several tens of thousands of microcircuits. According to the design and technological performance, microcircuits are divided into three groups: semiconductor. film and hybrid. In a semiconductor integrated circuit, all elements and interelement connections are made in the volume and on the surface of a semiconductor substrate, in a film integrated circuit, all elements and connections between them are made in the form of films. Currently, only passive elements of microcircuits - resistors, capacitors and inductances - are implemented using film technology methods. In a hybrid microcircuit, mounted discrete semiconductor devices or semiconductor integrated circuits are used as active electrical radio elements, and film resistors, capacitors, inductors and film conductors connecting them are used as passive elements.
The mechanical basis of such a microcircuit is a dielectric substrate. It performs the functions of a mechanical base, isolating elements from each other. heat sink. The substrates are available in the form of thin round or rectangular plates.
For semiconductor microcircuits, single-crystal semiconductor (silicon, gallium arsenide) and single-crystal dielectric (sapphire) substrates are used. A layer of semiconductor material is subsequently formed on them, in which microcircuit elements are created.
An indicator of the complexity of the microcircuit is the degree of integration K. which is characterized by the number of elements and components contained in it N: K = lgN. where K is rounded up to the nearest higher whole number. According to the degree of integration, microcircuits are divided into:
a) Small integrated circuits (MIS) are circuits of the 1st-2nd degree of integration, containing from several to 100 elements and components, which include one or more types of functional analog or logic elements. For example, logic elements AND, OR, NOT, triggers, amplifiers, filters, etc.
c) Medium integrated circuits [SIS] - circuits of the 2nd-3rd degree of integration, containing from several tens to 1000 elements and components, which include one or more identical functional units of electronic devices (register, counter, decoder, read-only memory device ).
d) Large integrated circuits (LSI) - circuits of the 3rd-4th degree of integration, containing from several hundred to 10,000 elements. which include one or more functional units (arithmetic logic unit, random access memory, reprogrammable read only memory).
e) Very large-scale integrated circuits (VLSI) are integrated circuits of 5-7 degrees of integration, which are a complete microelectronic product capable of performing the functions of equipment (for example, a microprocessor).

Fig.1-33 Semiconductor IC
Semiconductor microcircuits have the highest degree of integration. Figure 1-33 shows the semiconductor chip of the inverter and its circuit diagram. Elements for clarity are arranged in one line. All elements are placed in one p-type silicon wafer (Chapter 1.2.1). To exclude mutual influence, active and passive elements are placed in islands isolated from the substrate. From above, the substrate is protected by an insulating layer, on which conductive paths are applied, connecting the elements to each other.
For the production of microcircuits, a planar technology is used, which helps to simultaneously obtain a large number of microcircuits in a single technological process. Various structures are created on a single silicon wafer, forming a complete circuit, including active and passive elements.
The main semiconductor materials on which semiconductor microcircuits are currently manufactured are silicon and germanium. However, they are more promising. is silicon. SN is easily selective diffusion, has a higher resistance and allows you to expand the range of operating temperatures of microcircuits. An oxide film is easily formed on the silicon surface. which serves as a protective coating during a number of technological operations and protects the finished circuit from external influences.

Fig.1-34 Photomasks
After the plate surface is oxidized, it is necessary to select local areas on it, into which diffusion should be carried out. For this purpose, the method of photolithography is used. For the manufacture of microcircuits, several (5-20) different photomasks are needed. Figure 1-34 shows a set of photomasks for making a simple chip.
The described manufacturing process allows you to get several dozen chips of medium and high degree integration, i.e. as much as can be placed on one silicon wafer with a diameter of about 70 mm. The plate is divided into separate microcircuits. which are sealed in the housing. Pre-contact pads of the microcircuit are connected by conductors to the terminals of the case.
2 Amplifiers
2.1 Basic parameters
An electronic amplifier is a device that increases the voltage, current and power of an electrical signal by controlling the current of a powerful power source. Almost everywhere where electronic devices are used, electrical signals have to be amplified, and each specific device requires its own parameters and characteristics of the amplifier. It is almost impossible to produce ready-made amplifying devices with a very wide range that would satisfy any consumer. Therefore, the industry has mastered the production of a number of basic electronic amplifiers, the parameters and characteristics of which can be tuned by external circuits. A special place among them is occupied by operational amplifiers (op-amps), which are currently universal basic elements for building electronic amplifiers and other analog components of electronic equipment.
Parameters and characteristics of amplifiers based on op amps
The list of basic parameters of electronic amplifiers contains more than 30 items. One of the most important parameters is the voltage gain Ku - the ratio of the output voltage of the amplifier to the input voltage.
Ku \u003d Uout / Uin.
Parameters such as input impedance Rin and output impedance Rout allow you to evaluate the matching of the electronic amplifier with other electronic components connected to the amplifier.
The input impedance Rin allows you to calculate the effect of the input circuit of the amplifier on the electrical parameters of the device connected to it, and determine the power consumed by the input circuit of the amplifier.
Rin = dUin / dIin, where
dUin - voltage increment at the amplifier input;
dIin - current increment corresponding to dUin at the amplifier input.
A number of parameters, such as mixing voltage Ucm, input current Iin, non-linearity of the gain (dependence of K on the input voltage), maximum output voltage swing, and others, determine the difference between the properties of real amplifiers and an ideal linear amplifier and allow determining input signal amplification errors. For the same purposes, a number of amplifier characteristics are introduced - amplitude-frequency, phase-frequency, temperature, amplitude, etc., which make it possible to trace the dependence of the main parameters of the amplifiers on the signal parameters of external circuits and the environment.
2.2 Amplifier characteristics
2.2.1 Amplitude response

Fig.2-1 AX amplifier
AX determines the dependence of the amplitude of the output signal (current, voltage or power) on the amplitude of the input signal A 2 =F(A 1).
The working area of an amplifier is characterized by its dynamic range. D db \u003d 20Lg (U 1 max / U 1 min) - dynamic range, expressed in decibels (dB) (10 times - 20 dB, 100 times - 40 dB, 1000 times - 60 dB, etc.). The real dynamic range of the amplifier is about 60 dB.
2.2.2 Frequency response (AFC).

Fig.2-2 Gain versus frequency
The gain of the amplifier K changes with the frequency of the amplified signal f. Dependence K=F(f) contains information about both gain and frequency properties.

Fig. 2-3 Frequency response of various amplifiers
To eliminate the gain, the parameter M=K f /K 0 is introduced - the frequency distortion factor. The frequency properties are determined by the frequency response - this is the dependence M = F (f), where f is the frequency.

Figure 2-4 Typical Amplifier Frequency Response
The most common frequency response of the type shown in Fig.
Here f n is the lower limiting frequency, f in is the upper limiting frequency.
∆f=f in -f n - bandwidth.
If ∆f>>f 0 - broadband amplifier. When ∆f< The output signal of the amplifier can be shifted in phase with respect to the input. The dependence of this shift on frequency is the phase-frequency characteristic. In audio frequency amplifiers, the PFC is not used, since the ear does not distinguish between phase distortions. In video amplifiers, phase distortions are strictly normalized, because they lead to large image distortions. The transition characteristic is called the function h(t), where h(t)=U 2 /U 2∞ , t 0.9 -t 0.1 =t n - signal rise time δ i - emissions in HRP. For video amplifiers t n \u003d 0.1-1 ms In video technology, emissions of δ i 10% are permissible. R k is a load resistor, on it ∆I k creates a voltage drop ∆U R n = ∆I to R n, which is the output signal. R n \u003d (1 - 10) kOhm; R 1 , R 2 is a voltage divider that sets a small positive potential at the base with respect to the emitter. (100 - 300) kOhm; C 1 and C 2 are separating capacitors that separate the constant component of the signal at the input and output of the transistor. (1 - 5) uF; R E - emitter thermal stabilization resistor. Dramatically reduces the change in transistor current when it is heated. (500 Ohm - 2 kOhm); C E - capacitor, restores the gain of the variable component, which has decreased due to the inclusion of R E. (500 - 5000) uF; The applied input voltage U in causes a change in ∆U of the EB, which in turn causes a change in the collector current. And a change in the collector current causes a change in ∆U k. Since Rk can be chosen large enough, small changes in ∆Ube cause much larger changes in ∆U k, the signal is amplified. The parameters and characteristics of amplifiers can be adjusted using feedback. Feedback is the connection between the input and output of the amplifier, in which part of the energy from the output is fed to the input of the amplifier. The device that connects the output circuit of the amplifier to the input is called the feedback link. B is the transfer coefficient of the feedback link, it is usually set by passive circuits (resistors, capacitors, inductances). According to the method of connecting the input circuit of the feedback link B to the output terminals of the amplifier K, voltage feedback (Fig. 1) and current feedback (Fig. 2) are distinguished. According to the method of connecting the output circuit of the feedback link to the input terminals of the amplifier, there are serial (Fig. 3) and parallel (Fig. 4) feedback. Depending on whether the output voltage (current) is added to the input voltage (current) or subtracted, the feedback is respectively called positive (POS) or negative (OOS). Most often, environmental protection is used, because. PIC leads to instability. Designations in figures 1,2,3,4: Ug - signal source with voltage U; Rg - internal (output) resistance of the signal source; K - amplifier with gain K; B - feedback link with transmission coefficient B; Rn - load resistance. Any negative feedback (NFB) leads to a decrease in the gain and its stabilization. Serial voltage feedback increases Rin and decreases Rout. Serial current feedback increases Rin and increases Rout. Parallel voltage feedback reduces Rin and reduces Rout. Parallel current feedback reduces Rin and increases Rout. Kos - the gain of the amplifier covered by the serial voltage feedback is calculated by the formula: Kos \u003d K / (1 + K V) \u003d Uout / Ug Rin.os and Rout.os - the input and output impedance of the amplifier in this case is found from the ratios: Rin os \u003d Rin (1+ VK); (2) Rout os = Rout / (1 + BK). (3) For parallel voltage FOS, Rin is calculated by the formula: Rin os = R1 + 1 / Rin + (1 + HF) (4) Let us determine, for example, the gain of the amplifier K os, covered by a series OOS in terms of voltage Fig. 2-10. β=U OS /U 2 ; U OS \u003d U 2 β; K=U 2 /(U 1 -U OS)=U 2 /(U 1 -U 2 β); U 2 =KU 1 -KβU 2; U 2 =KU 1 /(1+Kβ); Hence: K os \u003d U 2 / U 1 \u003d K / (1 + Kβ) - gain of the amplifier covered by negative feedback, 1+Кβ=А – feedback depth; K os \u003d K / A. With the introduction of the CNF with a depth of A, the gain decreases by a factor of A, but at the same time, the instability of the gain caused by a change in temperature and supply voltage, a spread in the parameters of the circuit elements, as well as noise and alternating current background, decreases approximately by a factor of A. If A=2 - 5, then the feedback depth is considered small. If A=5 - 20 average feedback depth If A>20 deep feedback. The amplifier in Figure 2-11 is covered by a series voltage feedback. The transmission coefficient of the OS circuit is β=1 and K os =K/(1+Kβ)=K/(1+K)~1, so the circuit is called an emitter follower (EP). The EP is used as an output stage when the cable is connected as a load or the load resistance is low, as well as for decoupling individual blocks of the device. In such an amplifier, the transistor is connected according to the OB circuit. An example of an OS in a two-stage amplifier The amplifier Fig. 2-12 with a common serial OOS for voltage (using R os), in addition, the first stage is covered by a local serial OOS for current (using R e1). Capacitor C e1 is missing as it would short-circuit the feedback circuit. Sequential OOS in terms of voltage reduces R out and increases R in, A \u003d 1 + Kβ times. Parallel voltage feedback also reduces R out in A=1+Kβ times, but also reduces the input resistance. In order for the amplifier to be used in a wide variety of conditions, it is desirable that R in be as large as possible, and R out less (Fig. 2-13). Therefore, the most common series voltage feedback During operation, the transistor heats up, its current increases and normal operation is disrupted. To reduce this phenomenon, OOS is used. a) Thermal stabilization with the help of sequential OOS Fig. 2-11. In this circuit, Ue is the feedback voltage, Ueb \u003d U1-Ue is the control voltage. When heated, I k increases, U e increases, which leads to a decrease in U eb and I k., The collector current stabilizes. b) Thermal stabilization with the help of parallel NFB. Feedback is created using a resistor connected between the base and the emitter. With an increase in the collector current when the transistor is heated, the voltage drop across R to U R to \u003d I to R to increases, and the collector potential U to \u003d U pit -U R to decreases, which reduces the base current and, hence, the collector current. Therefore, the resistor R b with this inclusion creates an OOS that stabilizes the current of the transistor. These are amplifiers that amplify signals starting from the frequency f=0. Therefore, they never use capacitors. With the help of the divider R 1 , R 2 the potential U B =U A is set at U in =0, U out =U A -U B =0. UPTs are subject to the phenomenon of drift, which consists in the fact that U out slowly changes randomly, even when U in = const. Drift reasons: Instability of transistors and resistors with temperature changes, aging elements, Instability of power supplies. To reduce drift, highly stable resistors, low drift transistors, and voltage stabilization are used. There are circuit methods to reduce drift. One of them is the use of balanced amplification circuits. If transistors VT1 and VT2 have approximately the same drift, then over time U A and U B will change in the same way, and their difference U A -U B \u003d const, that is, the drift is significantly reduced. When an amplified signal is applied to VT1, U AB changes in antiphase with U input. Input VT2 can also be used to supply signals, then U AB changes in the same phase with U input. Therefore, U in2 is a direct input. U in1 is an inverted input. OA - an amplifier of electrical signals, made in the form of an integrated circuit with direct connections (DC) 2.4.1 and designed to perform various operations on analog signals when working in circuits with OOS. 1. Inverting op-amp: Since Ua=U2/Kou is very small, the input A is called virtual zero. We consider an ideal op-amp for which R in =∞, R out = 0, K U , oy =∞. I=E/(R 1 +R g) (1); I 2 =-I 1 (2); I 2 =U 2 /R OS (3); substitute (1) and (3) in (2), then we get: U 2 /R os =-E/(R 1 +R 2); K inv \u003d U 2 / E \u003d - R os / (R 1 + R g) (4) If R g \u003d 0, then | K inv | \u003d R OS / R 1 When setting R 2 =R 1 II R os significantly reduces the drift of the amplifier. R in, inv \u003d R 1 OA is covered by a parallel OOS for voltage, therefore R out. inv. =0. 2. Non-inverting op-amp: The op-amp is covered by a series OOS in terms of voltage (Fig. 2.18). In this circuit, R g does not affect the gain. Since the potentials at the inputs are very close, then E \u003d U R 1 \u003d U 2 R 1 / (R 1 + R os). Therefore, K is not inv =U 2 /E=(R 1 +R os)/R 1 =1+R os /R 1 =1+|K inv | In the second scheme, R os =0, so K is not inv =1 and it is a repeater. The amplifiers in question are called op-amps because they can perform various operations: 1) Addition of signals applied to the same input. 2) Subtraction of signals applied to different inputs. 3) By including non-linear elements (diodes) in the OS circuit, it is possible to perform logarithm and potentiation. 4) By including reactive elements (C) in the OS circuit, it is possible to perform integration and differentiation operations. The gain can be adjusted by simply changing R 1 and R oc (chapter 2.4.2.1) . Disadvantages of the method: the DC mode and the input resistance change, a non-linear adjustment is obtained when R 1 changes. 1).Discrete setting Ku;
The feedback depth is changed by a switch in the feedback circuit. 2) Smooth installation of K os (Fig. 2-20) Their frequency response (chapter 2.2.2) looks like Fig.2-21 Amplifiers are built using frequency-selective circuits, such as Wien's bridge. This R-C circuit is included in the positive feedback circuit of the op amp (Fig. 2-23) To avoid self-excitation, the gain of the feedback amplifier should be Kos<3.Для этого нужно очень точно устанавливать сопротивления R1 и Rос. Selective amplifier based on 2T-bridge Since the input current and the feedback current are equal (Figure 2-26), the output voltage is proportional to the input current. In this case, the load is included in the feedback circuit (Fig. 2-27). The scheme of Fig.2-28 allows you to adjust U stab2 \u003d-U stab1 R os /R 1 by changing R os. Flaw: small currents that can be drawn from the op-amp. To increase the current at the output of the circuit, an emitter follower (Chapter 2.3.2) is installed on a powerful transistor. With an increase in the load current R n (for example, in the event of a short circuit), the current VT1 will increase to an unacceptable value, but at the same time the voltage across the resistor R increases, it opens VT2. Through VT2, the VT1 base turned out to be connected to the emitter and VT1 closes. The voltage on R ext tends to zero. VT2 closes. Again, overload occurs and the process is repeated, as a result, U stub has the form (1). For normal operation of the circuit in Fig. 2-31, it is necessary that the current in the zener diode circuit I stub >= 1.5 I oy. The disadvantage of the scheme:: U>U 1 +U 2 since on R limit. additional voltage drops. For the normal operation of this circuit, Figure 2-32, it is necessary: I R1, R2 >=10I oy Rectifiers are designed to convert AC voltage (current) to DC. They are used to power almost all devices based on semiconductor and integrated elements, in industrial installations for electric welding and metal smelting, in technology with electrolysis processes, in electric drives of various Vehicle etc. Depending on the number of phases, single-phase and multi-phase (usually three-phase) rectifiers are distinguished. In terms of power, rectifiers are divided into small, medium and high power rectifiers. Low power rectifiers are usually single-phase, medium and high power are three-phase. A generalized block diagram of the low power rectifier is shown in Figure 3-1. The AC mains voltage is converted with the help of a transformer Tr into a voltage of the required value U2 and then supplied to the block B, assembled on semiconductor or any other diodes (valves), at the output of which the voltage Ub becomes unipolar, but varies in value over time (pulsating ), Often after block B they put a filter F, Usually consisting of passive elements of type C and L, and less often of active elements - transistors, which pass well only the constant component of the rectified voltage into the load. With correctly selected filter elements F, the voltage Uf at its output has very small ripples. If the valve converter B is assembled on controlled elements (thyristors, transistors), then a system is added to it that controls the processes of opening and closing valves (CS). The rectified voltage in the load can vary greatly both due to daily fluctuations in the AC mains voltage, and as a result of changes in the magnitude of the load current. To ensure the required voltage stability at the load, a rectified voltage stabilizer (St) is used. The operational properties of rectifiers are characterized by the following main quantities: A. The average value of the rectified voltage and current (U 0, I 0). b. Efficiency factor (efficiency). V. The ripple coefficient p, determined by the ratio of the amplitude of the first harmonic U m 1 of the rectified voltage to the value of its average component U 0 p=U m 1 / U 0 . G. External characteristic - the dependence of the output (rectified) voltage on the amount of current consumed by the load U 0 \u003d f (I n). d. Regulating characteristic - the dependence of the rectified voltage on the control angle (on time) of the valves. Rectification is based on the one-way conductance (gate properties) of semiconductor diodes (chapter 1.3). A diagram of a half-wave (single-cycle) rectifier and diagrams of the rectified voltage Un and current In are shown in Fig. 3-2. The power transformer Tr is necessary to obtain the voltage of the required value, and in radio electronics, and to separate the load circuits Rn and the AC network. Diode D (Fig. 2-34a) conducts current in that half-cycle of the alternating voltage when the potential Ub > Ua. The current flows through the circuit B - Rn - D - A. During the second half-cycle of the alternating voltage Ua > Ub, there is practically no current closing diode in the circuit. The pulsating current In creates a pulsating voltage Un of the same form on the load (Fig. 3-2b). It is shown in Fig. 3-3a, it allows you to get the current flowing in the load during both half-cycles of the alternating voltage. This is achieved by using two secondary windings AB and BC and two diodes. Let in the first half-cycle Ua > Ub > Us. Then the current flows through the circuit A - D1 - Rn - B, as in the case of single-half-wave rectification. During the second half-cycle Ua< Ub < Uс и ток протекает по цепи С - D2 – Rн - В. Направление тока через нагрузку остается неизменным. Форма выпрямленного тока и напряжения (временная диаграмма) в этом случае показана на рис.3-3в. The ripple frequency is equal to twice the frequency of the alternating voltage. The secondary winding of the rectifier transformer fig3-3a has twice as many turns as the transformer fig3-2a. This increases the size and cost of the rectifier unit. There is no such drawback in a full-wave bridge rectifier (Fig. 3-3b). When the potential Ua\u003e Ub, the current flows through the circuit A-D1-Rn-D3-B. During the second half-cycle, Ub > Ua and the current path is B-D4-Rн-D2-A. The direction of the current through Rn remains unchanged and, thus, full-wave rectification is carried out. The timing diagrams of the rectifier fig.3-3c are the same as the rectifiers fig.3-3a. As the simplest filter, a capacitor C of a sufficiently large capacity is used, connected in parallel with the load. Replacing the transformer together with the valves (for example, in the circuit of Fig. 2a) with an equivalent generator with voltage Uv and internal resistance r x, we obtain the equivalent circuit of the rectifier (Fig. 3-4a). In it, r x is determined by the total resistance of the valves and windings of the transformer, U in - the value of the rectified voltage in the idle mode (Rn = oo). From Kirchhoff's laws it follows that the voltage at the load (terminals cd) will be equal to: Un = Uv-(Is + In)r x , (1) where Ic is the charging current of the capacitor, In is the load current. Figure 3-4 also shows waveforms for a half-wave (upper) and for a full-wave (lower) rectifier. During the time t1-t2, when the value of U2 increases, the capacitor Co is charged by the current Ic, and in the interval t2-t3 it is partially discharged through Rn, since in this case the diodes of the valve block are closed and do not allow it to be discharged through the transformer winding. Such a filter significantly reduces the ripple of the rectified voltage. The quality of smoothing is characterized by the coefficient of ripple p, expressed as a percentage p = (Um / Uo) *100% , where Um is the amplitude of the first harmonic, Uo is the constant component of the rectified voltage. The capacitive filter reduces ripple by up to 5-15% compared to 157% and 66.7% respectively for half-wave and full-wave rectifiers without a filter. The value of the ripple factor with a capacitive filter is determined by the formulas p \u003d 600 Io / UoCo - for a single-wave rectifier and p \u003d 300 Io / UoCo - for full-wave. Here Co is in uF, Io is in mA, Uo is in V. To power electronic equipment, p = 0.05–1% or less is allowed, therefore more complex filters are used. The magnitude of the pulsations is also significantly reduced by the St. stabilization unit (Fig. 3-1). Calculation formulas for rectifiers 1) Diode calculation: Imax=7I 0 ,Uarr=3U 0
2) Transformer calculation: U 2 \u003d 0.75U 0 + I 0 (R i + R tr) / 265 R i – diode internal resistance R iGe =500/I0(mA), R iSi =100/I0(mA). R tr - resistance of the internal windings of the transformer R tr \u003d 500U 0 / (I 0 (U 0 I 0) 1/4), Secondary winding current: I 2 \u003d 2I 0 + 12U 0 / (R i + R tr) 3) Capacitor calculation: U C 0 \u003d 1.2U 0 p 0 \u003d 600I 0 / U 0 C 0; C 0 \u003d 600I 0 / U 0 p 0. Full-Wave Mid-Point Rectifier 1) Diode selection: Uobr=3U 0 , Imax=3.5I 0 2) Transformer selection: U 2 \u003d 0.75U 0 + I 0 (Ri + Rtr) / 530 Rtr=1000/I 0 (U 0 I 0) 1/4 I2=I0+12U0/(Ri+Rtr) 3) Calculation of the capacitor: C 0 \u003d 300I 0 / U 0 P 0 (%); U C 0 \u003d 1.2U 0 Full Wave Bridge Rectifier 1) Uobr=1.5U 0 , Imax=3.5I 0 2) U 2 \u003d 0.75U 0 + I 0 (2R i + R tr) / 530; R tr \u003d 830 / I 0 (U 0 I 0) 1/4; I 2 \u003d 21/2 I 0 +16.6U 0 / (2R i + R tr) 3) C 0 \u003d 300I 0 / U 0 p 0 (%); U C0 \u003d 1.2U 0 Calculation for L-shaped filter: a)LC
– filter
For half-wave For full-wave rectifier LC=10r 0 /r LC=2.5r 0 /r b)RC
– filter
Filter elements are determined from expressions: For half-wave to full-wave rectifier RC=3000r 0 /r RC=1500r 0 /r The rectifier output voltage is unstable. For example, with an increase in the consumed current I n, the filter capacitor C in is discharged to a greater extent in the time interval t2-t3 (Fig. 3-4), therefore, to recharge it during the time t1-t2, a larger charging current Ic is required. But then from equation (1) it can be seen that the voltage loss across the resistance r z will increase and U n will decrease. The graph in Fig. 3-8 shows the external characteristics of the rectifier without a filter - Ic = 0 and with a capacitive filter - Ic > 0. The characteristics are built on the basis of equation (1), taking into account the fact that the valves have a resistance that depends nonlinearly on the flowing current. So that the value of U n practically does not change with increasing current In, a voltage stabilizer is introduced into the rectifier. At low load currents and low stability requirements for Ust, the simplest parametric stabilizers on a silicon zener diode (Chapter 1.4) (Fig. 3-9a) are used. The current-voltage characteristic of a silicon zener diode (Fig. 3-9b) has a section mn, on which, when the current changes from I min to I max, the voltage remains almost constant. If necessary, increase Ust, the zener diodes are connected in series. The stabilizer fig.3-9a reduces the relative voltage changes by 5-10 times. But it is impossible to change the value of Ust in a parametric stabilizer. It is determined by the selected zener diode. If such stabilization does not meet the requirements, then semiconductor compensation voltage stabilizers are used. Figure 3-9a shows the principle of stabilization, based on compensation for voltage changes in the load by changing the resistance value of the variable resistor R1, connected in series with the load Rn. For the circuit under consideration, we can write the equation Un \u003d Uf - I 1 * R 1, (3) that is, the voltage Un at the load is less than the rectified voltage (at the filter output) Uf by the amount of voltage drop across the variable resistor R 1 . By changing the resistance value, you can adjust the voltage Un. For any change in the rectified voltage dUf, by analogy with equation (3), we can write that dUн \u003d dUf - dI 1 * R 1. (4) Therefore, setting the value of R 1 always so that the equality dUf \u003d dI 1 *R 1 is fulfilled, we get dUn = 0, that is, the voltage at the load will be constant. To automate the process of stabilizing the voltage Un, a high-power transistor VT1 is used as a variable resistor R1 (Fig. 3-10b), and the transistor VT2 controls its resistance. The collector current VT2 changes with a change in voltage Un. Therefore, the base current of the transistor VT1 changes and, consequently, its resistance. The parametric stabilizer R4-V3 plays an auxiliary role, providing a reference (constant) voltage at the emitter VT2, with which the voltage changes at the load are compared, coming to the base VT2 through the divider R1-RЗ. The scheme works as follows. Let, for some reason, the voltage Un begin to decrease (potential fn relative to point 3 increases). Then the base potential VT2 also increases relative to the emitter (it becomes less negative), and its base current Ib2 decreases. In this case, the collector current Ik2 \u003d b2 * Ib2 (b2 is the current transfer coefficient of the transistor VT2) will reduce the potential of the base of the transistor VT1 (Ub1 \u003d Uk2 ~ Uf - Ik2 * R5) and, therefore, reduce the resistance of the transistor VT1. At the same time, the voltage U1 \u003d inevitably decreases I1*Rv1 (see Fig. 3-10b), and the voltage in the load Un = Uf - I1*Rv1 will practically remain unchanged. External characteristics of stabilized rectifiers are shown in Fig.3-11. Voltage constancy Un is maintained by parametric (curve 2) and compensation (curve 3) stabilizers 1 - without stabilizer; 2 - with parametric stabilizer; 3 - with a transistor stabilizer of a compensation type. Up to a certain value of the maximum load current, depending on the type of semiconductor devices used. The compensation type stabilizer smooths out ripples very well, if they are not too large at the rectifier output and the input voltage drop does not take the regulator out of its normal operating region. > Disadvantages of the above stabilizers: 1) Low efficiency, not exceeding 50%. 2) Large dimensions of the capacitor and inductance in the filter. These shortcomings are removed when using a pulse (key) stabilizer. In this stabilizer, the VT transistor is put in the key mode: The PWM generator provides pulse-width modulation, in which the width of the generated pulses Ug is proportional to the control voltage Ucontrol. The working process of a switching stabilizer 1) During the pulse U control, the transistor VT opens, the capacitance C is recharged through the inductance 2) VT1 closes, inductance and capacitance give energy to the consumer. Diode VD is installed to close the reverse current of the inductance through the capacitance and load. The PWM generator generates a sequence of pulses to the base VT, the width of which depends on U out. The pulse duration t and \u003d K (U op -U out R 1 / (R 1 + R 2)) 3) If, for example, the output voltage decreases, then the duration of the pulses increases. This increases the energy stored in the inductance and the output voltage is maintained constant. The clock frequency is approximately 20 kHz. The capacitor is "feeded" quite often, so its capacity is much less than when using a continuous stabilizer. GSK is a device made on the basis of autonomous self-oscillating circuits, in which a sinusoidal change in voltage and current occurs without the application of an additional periodic signal. This is the conversion of direct current energy into the energy of sinusoidal electrical oscillations. Type GeneratorL-
C:
Generation occurs due to positive feedback between the collector and base of the transistor through mutual inductance between the coils. Fluctuations occur when two conditions are met: 1) The amplitude condition, which is satisfied if the coils Lsv and L are located close enough. 2) Phase condition. Coil Lsv must be turned on so that the resulting feedback is positive. Then there are fluctuations with frequency ω 2 LC=1; Therefore ω=1/(LC) 1/2 ; f=1/2π(LC) 1/2 Generators with frequency f>=150 kHz are built according to this scheme. For lower frequencies, RC oscillators are used. Zone theory solids 1. Metals conduct electricity well. Dielectrics (insulators) do not conduct current well. Electrical conductivity of metals 10 6 – 10 4 (Ohm×cm) -1 Conductivity of dielectrics less than 10 -10 (Ohm×cm) -1 Solids with intermediate electrical conductivity are called semiconductors. 2. The difference between semiconductors and metals is manifested in the nature of the dependence of electrical conductivity on temperature. With decreasing temperature, the conductivity of metals increases, and for pure metals tends to infinity when approaching absolute zero. In semiconductors, on the contrary, with decreasing temperature, the conductivity decreases, and near absolute zero, the semiconductor becomes an insulator. 3. Not classic electron theory electrical conductivity, nor quantum theory, based on the free fermian model, cannot answer the question why some bodies are semiconductors, while others are conductors or dielectrics. 4. To answer the question, it is necessary to consider the question of the interaction of valence electrons with atoms of the crystal lattice using the methods of quantum mechanics. 5. Solving the Schrödinger equation with the number of variables of order 10 23 is mathematical problem hopeless difficulty. Therefore, the modern quantum theory of solids is based on a number of simplifications. Such a theory is the theory of solids. The name is associated with the characteristic grouping of the energy levels of electrons in crystals into zones of levels. The band theory is based on the following assumptions: 1) When studying the motion of valence electrons, the positive ions of the crystal lattice, due to their large mass, are considered as stationary sources of the field acting on the electrons. 2) The arrangement of positive ions in space is considered to be strictly periodic: they are located at the nodes of the ideal crystal lattice of a given crystal. 3) The interaction of electrons with each other is replaced by some effective force field. The problem is reduced to considering the motion of an electron in a periodic force field of a crystal. The potential energy of the electron U(r) changes periodically. §2. The simplest model of a crystalline body This is a one-dimensional Kronig-Penny model, the periodic electric field of the positive ions of the crystal is approximated by a "toothed wall" type potential. The figure shows the alternation of potential wells and barriers. Solution of the Schrödinger equation for a potential well: Potential Barrier Solution: Where , X n - the coordinate is counted from the beginning of the nth section. They write down for each well and barrier, then they "stitch" the solutions and get the basic equation for determining the energy levels in the periodic field of the crystal. Where Graphical representation of the solution to the Schrödinger equation according to Kronig–Penny. Cos k′a can vary from –1 to +1. We drew parallel lines of the abscissa axis and find the points of intersection of these lines with the graph, omit the perpendiculars and find the roots of equation (3). These areas are marked with bold lines. Thus, the admissible values of E(k) have a discrete character (zonal). If the axis (Ka) is turned to a vertical position, then we get a picture of the location of the energy zones, allowed and prohibited. In Fig.4, the energy spectrum of electrons in a crystal has a band structure. L is the length of the chain ring. Wave vector values . α is the lattice constant. The band derived from the valence levels of the atoms that form the crystal is called the valence band. The bands originating from the inner levels are always completely filled with electrons. Partially filled or unfilled may be the outer valence level (conduction band). The 3S electrons are the weakest bound. When a solid body is formed from individual atoms, the wave functions of these electrons overlap. A solid body of four atoms will have a total of four levels distributed over a certain energy range. For example: in the ground state of a hydrogen atom, an electron can be in one of two states - with a spin up or down. There are eight possible states in a system of four protons. But if you add three more electrons to make four hydrogen atoms, then four states will be occupied, and there will be two states for each electron. The effect of the approach of atoms is manifested in a change in the energy of individual states where is the energy of an isolated atom, are the energy changes associated with the influence of the corresponding protons 2, 3, 4. R is the distance between atoms. The effect of the approach of atoms is manifested in an increase total number levels. A real body contains about 10 23 individual levels, which are continuously distributed within a certain interval, forming a zone of allowed energy values (Fig. 9). The same situation basically takes place for the valence electrons of any atom. In solid sodium, the 3S - electron zone is external, half filled. The upper boundary of the filled levels falls in the middle of the zone. An electron can move to a higher free level in this band due to thermal or electrical excitation. Therefore, solid sodium has good electrical and thermal conductivity. Figure 10 shows the band structure of conductors (sodium). The upper zone is a partially filled zone. The lower zones are filled with electrons. If the number of energy levels in the zone more number electrons in it, then the electrons are easily excited, thereby providing conduction, but if all levels in the zone are filled, then conduction is impossible or difficult. For example: in silicon, germanium, carbon (diamond) on the P-shell, there are two electrons and a mixed configuration of S and P-orbitals arises (an orbital is a wave function that describes a given quantum state), which makes the four-atom configuration shown in Fig. fig.11 (energy of Coulomb repulsion of electrons is minimal). The wave functions of S and P - electrons form one completely empty hybrid SP - zone and one filled hybrid SP - zone. The filled and empty bands are separated by a fairly significant energy interval or band of forbidden energy values. For insulators, the typical value of the band gap is ~5 eV and more. The band gap for semiconductors (Germany 0.67 eV, silicon 1.12 eV) is within 0.1 ¸ 3 eV. Semiconductors and insulators differ from each other only in the band gap. § Bloch's theorem Bloch's theorem states that the eigenfunctions of the wave equation with a periodic potential have the form of the product of the plane wave function On the function , which is a periodic function in the crystal lattice: The index in indicates that this function depends on the wave vector . The wave function is called the Bloch function. Solutions of the Schrödinger equation of this kind consist of traveling waves, from such solutions one can compose a wave packet, which will represent an electron freely propagating in a periodic potential field created by ionic cores. Wave packet shape at t=0 for de Broglie waves. The amplitude is indicated by a dashed line, the wave is a solid line. The motion of a monochromatic plane wave along the X axis can be described by the function The speed of wave propagation can be found as the speed of displacement of the constant phase. If time changes by ∆t, then in order for condition (2) to be satisfied, the coordinate must change by ∆x, which can be found from the equality those. Hence the propagation velocity of the constant phase, called the phase velocity: The phase velocity of photons (m 0 = 0) is equal to the speed of light The phase velocity of an electron moving at a speed V can be written those. it becomes greater than the speed of light, since V< с. Это говорит о том, что фазовая скорость не может соответствовать движению частицы или же переносу какой-либо энергии. The real process cannot be purely monochromatic (k = const). It always has a certain width, i.e. consists of a set of waves with close wave numbers, and at the same time, frequencies. Using a set of waves, it is possible to construct a wave packet whose amplitude is nonzero only in a small region of space, which is associated with the location of the particle. The maximum amplitude of the wave packet will propagate at a speed that is called the group velocity. Amplitude B of the wave packet where A is the constant amplitude of each of these waves. B propagates at a speed For photons (m 0 = 0) For de Broglie waves those. the group velocity coincides with the velocity of the particle. At points The square of the amplitude vanishes. Wave packet localization region where is the width of the wave packet. where is the spreading time of the wave packet. Heisenberg uncertainty relations. The smaller, the wider. For a monochromatic wave where the amplitude in the whole space has the same value, i.e. the superposition of a particle (one-dimensional case) in the whole space is equiprobable. This generalizes to the three-dimensional case as well. For the nonrelativistic case (m = m 0), the spreading time of the wave packet if m = 1r, then the melting time is extremely long. In the case of an electron, m 0 ~ 10 -27 g those. to describe an electron in an atom, we must use the wave equation, because the wave packet spreads almost instantly. The photon wave equation contains the second derivative with respect to time, since a photon is always a relativistic particle. The movement of an electron in a crystal Law of motion compared to where m* is the effective mass, it takes into account the joint action of the potential field and external force per electron in a crystal. in the conduction band, In the valence band In the valence band, but in the band of germanium and silicon, there are heavy and light holes. Effective masses are always expressed as fractions of the true mass m 0 = 9 10 -28 g The effective mass is a tensor quantity, in various directions it is different, which is a consequence of the anisotropic properties of the crystals. E k is the equation of an ellipsoid of revolution and is described by two values of the masses and Energy spectrum of electrons and holes in coordinates E and K Electron momentum Holes - quasi-particles with lower energy are located at the top of the valence band and increase their energy, moving along the energy scale deep into the valence band. For holes and electrons, the energies are counted in opposite directions. Electrons and holes that have a wave vector can collide with other particles or fields as if they had momentum It is called a quasi-momentum. Designation Name Phonons scatter X-rays and neutrons. momentum in quantum mechanics operator answers. those. plane wave Ψ k is an eigenfunction of the momentum operator, and the eigenvalues of the momentum operator are The Fermi energy is defined as the energy of the electrons at the highest filled level where n F is the quantum number of the highest occupied energy level. where N is the number of electrons in the volume Energy is a quadratic function of the quantum number n F . The wave functions satisfying the Schrödinger equations for a free particle in a periodic field are traveling plane waves: provided that the components of the wave vector take the values similar sets for K y and K z . Any vector component has the form n is a positive integer or a negative number. Components are quantum numbers along with quantum numbers setting the direction of the back. those. energy eigenvalues of states with a wave vector where is the energy of an electron with a wave vector ending at the surface of the sphere. Each triple of quantum numbers K x , K y , K z corresponds to a volume element in K-space with the value . therefore, in a sphere of volume, the number of points describing allowed states is equal to the number of cells of volume , and therefore the number of allowed states is where the factor 2 on the left side takes into account two possible values of the spin quantum number for each allowed value The total number of states is equal to the number of electrons N. The radius of the Fermi sphere K F depends only on the concentration of particles and does not depend on the mass m The Fermi energy can be defined as the energy of such quantum states, the probability of filling them with a particle is equal to 1/2. if E \u003d E F, then But absolute zero temperature is understood as a limit keeping in mind that absolute zero is not achievable and plus the Pauli principle. Usually, systems are considered not only at T = 0, but also at any temperature, if the boundary energy For such systems, where the dependence of E F on temperature can be neglected and considered There are tables of Fermi surface parameters for a number of metals calculated for the free electron model for room temperature (T = 300 0 K). The electron concentration is determined by the product of the valence of the metal by the number of electrons in 1 cm 3. then we get: Or if , For example: Li Valence - 1, *r 0 is the radius of the sphere containing one electron. L n - Bohr radius 0.53 × 10 -8 cm. * Wave vector K F = 1.11×10 8 cm -1 ; Fermi velocity V F = 1.29×10 8 cm/s; Fermi energy Fermi temperature T F has nothing to do with the temperature of the electron gas. We define - the number of states per unit energy interval, the part called the density of states at The density of states is: Option 5 No. 2. The number of electrons with kinetic energy from Е F /2 to Е F is determined by the relation Similarly: The same result can be obtained from in a simpler form: With an accuracy of the order of unity, the number of states per unit energy interval near the Fermi energy is equal to the ratio of the number of conduction electrons to the Fermi energy. conclusions 1. Effective masses: germanium silicon those. in the valence band of germanium and silicon there are heavy and light holes. The valence bands consist of three subbands. 3. The diversity of properties of solids is evidence of the diversity of quasiparticles. 4. Until recently, it was believed that electrons are similar to each other. When one wants to emphasize the difference between iron electrons and copper electrons, one says that they have different Fermi surfaces. At the World Exhibition in Brussels, the building pays tribute to the age of physics. Represents the correct system of interconnected spheres, within which there are exhibition spaces. Each of which (sphere) represents an iron ion that has lost one electron. This is the Fermi level surface. Each metal has only its own inherent shape of the Fermi surface; it limits the region of momentum space occupied by conduction electrons at absolute zero. These are business cards of various metals. ... th zone. For those, in which the width of the fenced zone does not exceed 1 eV, already at room temperature in the conductivity zone, a sufficient number of electrons appear, and in the valence zone - vacancies, so that a high electrical conductivity can be increased. Such bodies are obviously called napіvprovіdnikami. Let’s make it clear that I’ve split the solid bodies of another group, into dielectrics and heaters, we’ll cleanly understand. At... Around the world. If in 1900 about 8 thousand tons of light metal were produced per year, then in a hundred years the volume of its production reached 24 million tons. 2. Metallic conductive and semiconductor materials, magnetic materials 2.1 Classification of electrical materials Electrical materials are a combination of conductive, electrical insulating, magnetic and ... Types of processing in the manufacture of the necessary products from them. Therefore, different materials have to be chosen for different applications. Electrical insulating materials form the most numerous section of electrical materials in general; the number of individual types of specific electrical insulating materials used in the modern electrical industry amounts to many thousands ... 1. Metals conduct electricity well. Dielectrics (insulators) do not conduct current well. Electrical conductivity of metals 10 6 – 10 4 (Ohm×cm) -1 Conductivity of dielectrics less than 10 -10 (Ohm×cm) -1 Solids with intermediate electrical conductivity are called semiconductors. 2. The difference between semiconductors and metals is manifested in the nature of the dependence of electrical conductivity on temperature. With decreasing temperature, the conductivity of metals increases, and for pure metals tends to infinity as it approaches absolute zero. In semiconductors, on the contrary, with decreasing temperature, the conductivity decreases, and near absolute zero, the semiconductor becomes an insulator. 3. Neither the classical electronic theory of electrical conductivity nor the quantum theory based on the model of free fermians can answer the question why some bodies are semiconductors, while others are conductors or dielectrics. 4. To answer the question, it is necessary to consider the question of the interaction of valence electrons with atoms of the crystal lattice using the methods of quantum mechanics. 5. Solving the Schrödinger equation with the number of variables on the order of 10 23 is a mathematical problem of hopeless difficulty. Therefore, the modern quantum theory of solids is based on a number of simplifications. Such a theory is the theory of solids. The name is associated with the characteristic grouping of the energy levels of electrons in crystals into zones of levels. The band theory is based on the following assumptions: 1) When studying the motion of valence electrons, the positive ions of the crystal lattice, due to their large mass, are considered as stationary sources of the field acting on the electrons. 2) The arrangement of positive ions in space is considered to be strictly periodic: they are located at the nodes of the ideal crystal lattice of a given crystal. 3) The interaction of electrons with each other is replaced by some effective force field. The problem is reduced to considering the motion of an electron in a periodic force field of a crystal. The potential energy of the electron U(r) changes periodically. §2. The simplest model of a crystalline body This is a one-dimensional Kronig-Penny model, the periodic electric field of the positive ions of the crystal is approximated by a "toothed wall" type potential. The figure shows the alternation of potential wells and barriers. Solution of the Schrödinger equation for a potential well: Potential Barrier Solution: Where , X n - coordinate is counted from the origin n th site. They write down for each well and barrier, then they "stitch" the solutions and get the basic equation for determining the energy levels in the periodic field of the crystal. Where In Fig.4, the energy spectrum of electrons in a crystal has a band structure. L is the length of the chain ring. Wave vector values . α is the lattice constant. The band derived from the valence levels of the atoms that form the crystal is called the valence band. The bands originating from the inner levels are always completely filled with electrons. Partially filled or unfilled may be the outer valence level (conduction band). The 3S electrons are the weakest bound. When a solid body is formed from individual atoms, the wave functions of these electrons overlap. A solid body of four atoms will have a total of four levels distributed over a certain energy range. For example: in the ground state of a hydrogen atom, an electron can be in one of two states - with a spin up or down. There are eight possible states in a system of four protons. But if you add three more electrons to make four hydrogen atoms, then four states will be occupied, and there will be two states for each electron. The effect of the approach of atoms is manifested in a change in the energy of individual states where is the energy of an isolated atom, are the energy changes associated with the influence of the corresponding protons 2, 3, 4. R is the distance between atoms. The effect of the approach of atoms manifests itself in an increase in the total number of levels. A real body contains about 10 23 individual levels, which are continuously distributed within a certain interval, forming a zone of allowed energy values (Fig. 9). The same situation basically takes place for the valence electrons of any atom. In solid sodium, the 3S - electron zone is external, half filled. The upper boundary of the filled levels falls in the middle of the zone. An electron can move to a higher free level in this band due to thermal or electrical excitation. Therefore, solid sodium has good electrical and thermal conductivity. Figure 10 shows the band structure of conductors (sodium). The upper zone is a partially filled zone. The lower zones are filled with electrons. If the number of energy levels in the zone is greater than the number of electrons in it, then the electrons are easily excited, thereby providing conduction, but if all levels in the zone are filled, then conduction is impossible or difficult. For example: in silicon, germanium, carbon (diamond) on the P-shell, there are two electrons and a mixed configuration of S and P-orbitals arises (an orbital is a wave function that describes a given quantum state), which makes the four-atom configuration shown in Fig. fig.11 (energy of Coulomb repulsion of electrons is minimal). The wave functions of S and P - electrons form one completely empty hybrid SP - zone and one filled hybrid SP - zone. The filled and empty bands are separated by a fairly significant energy interval or band of forbidden energy values. For insulators, the typical value of the band gap is ~5 eV and more. The band gap for semiconductors (Germany 0.67 eV, silicon 1.12 eV) is within 0.1 ¸ 3 eV. Semiconductors and insulators differ from each other only in the band gap. § Bloch's theorem Bloch's theorem states that the eigenfunctions of the wave equation with a periodic potential have the form of the product of the plane wave function On the function , which is a periodic function in the crystal lattice: The index in indicates that this function depends on the wave vector . The wave function is called the Bloch function. Solutions of the Schrödinger equation of this kind consist of traveling waves, from such solutions one can compose a wave packet, which will represent an electron freely propagating in a periodic potential field created by ionic cores. Wave packet shape at t=0 for de Broglie waves. The amplitude is indicated by a dashed line, the wave is a solid line. The motion of a monochromatic plane wave along the X axis can be described by the function The speed of wave propagation can be found as the speed of displacement of the constant phase. If time changes by ∆t, then in order for condition (2) to be satisfied, the coordinate must change by ∆x, which can be found from the equality those. Hence the propagation velocity of the constant phase, called the phase velocity: The phase velocity of photons (m 0 = 0) is equal to the speed of light The phase velocity of an electron moving at a speed V can be written those. it becomes greater than the speed of light, since V< с. Это говорит о том, что фазовая скорость не может соответствовать движению частицы или же переносу какой-либо энергии. The real process cannot be purely monochromatic (k = const). It always has a certain width, i.e. consists of a set of waves with close wave numbers, and at the same time, frequencies. Using a set of waves, it is possible to construct a wave packet whose amplitude is nonzero only in a small region of space, which is associated with the location of the particle. The maximum amplitude of the wave packet will propagate at a speed that is called the group velocity. Amplitude B of the wave packet where A is the constant amplitude of each of these waves. B propagates at a speed For photons (m 0 = 0) For de Broglie waves those. the group velocity coincides with the velocity of the particle. At points The square of the amplitude vanishes. Wave packet localization region where is the width of the wave packet. where is the spreading time of the wave packet. Heisenberg uncertainty relations. The smaller, the wider. For a monochromatic wave where the amplitude in the whole space has the same value, i.e. the superposition of a particle (one-dimensional case) in the whole space is equiprobable. This generalizes to the three-dimensional case as well. For the nonrelativistic case (m = m 0), the spreading time of the wave packet if m = 1r, then the melting time is extremely long. In the case of an electron, m 0 ~ 10 -27 g those. to describe an electron in an atom, we must use the wave equation, because the wave packet spreads almost instantly. The photon wave equation contains the second derivative with respect to time, since a photon is always a relativistic particle. The movement of an electron in a crystal where m* is the effective mass, it takes into account the joint action of the potential field and external force on an electron in a crystal. in the conduction band, In the valence band In the valence band, but in the band of germanium and silicon, there are heavy and light holes. Effective masses are always expressed as fractions of the true mass m 0 = 9 10 -28 g The effective mass is a tensor quantity, it is different in different directions, which is a consequence of the anisotropic properties of crystals. E k is the equation of an ellipsoid of revolution and is described by two values of the masses and Energy spectrum of electrons and holes in coordinates E and
K Electron momentum Holes - quasi-particles with lower energy are located at the top of the valence band and increase their energy, moving along the energy scale deep into the valence band. For holes and electrons, the energies are counted in opposite directions. Electrons and holes that have a wave vector can collide with other particles or fields as if they had momentum It is called a quasi-momentum. Phonons scatter X-rays and neutrons. In quantum mechanics, the momentum corresponds to the operator . those. plane wave Ψ k is an eigenfunction of the momentum operator, and the eigenvalues of the momentum operator are The Fermi energy is defined as the energy of the electrons at the highest filled level where n F is the quantum number of the highest occupied energy level. where N is the number of electrons in the volume Energy is a quadratic function of the quantum number n F . The wave functions satisfying the Schrödinger equations for a free particle in a periodic field are traveling plane waves: provided that the components of the wave vector take the values similar sets for K y and K z . Any vector component has the form n is a positive or negative integer. Components are quantum numbers along with quantum numbers setting the direction of the back. those. energy eigenvalues of states with a wave vector where is the energy of an electron with a wave vector ending at the surface of the sphere. Each triple of quantum numbers K x , K y , K z corresponds to a volume element in K-space with the value . therefore, in a sphere of volume, the number of points describing allowed states is equal to the number of cells of volume , and therefore the number of allowed states is where the factor 2 on the left side takes into account two possible values of the spin quantum number for each allowed value The total number of states is equal to the number of electrons N. The radius of the Fermi sphere K F depends only on the concentration of particles and does not depend on the mass m The Fermi energy can be defined as the energy of such quantum states, the probability of filling them with a particle is equal to 1/2. if E \u003d E F, then But absolute zero temperature is understood as a limit keeping in mind that absolute zero is not achievable and plus the Pauli principle. Usually, systems are considered not only at T = 0, but also at any temperature, if the boundary energy For such systems, where the dependence of E F on temperature can be neglected and considered There are tables of Fermi surface parameters for a number of metals calculated for the free electron model for room temperature (T = 300 0 K). The electron concentration is determined by the product of the valence of the metal by the number of electrons in 1 cm 3. then we get: Or if , For example: Li Valence - 1, *r 0 is the radius of the sphere containing one electron. L n - Bohr radius 0.53 × 10 -8 cm. * Wave vector K F = 1.11×10 8 cm -1 ; Fermi velocity V F = 1.29×10 8 cm/s; Fermi energy Fermi temperature T F has nothing to do with the temperature of the electron gas. We define - the number of states per unit energy interval, the part called the density of states at The density of states is: Option 5 No. 2. The number of electrons with kinetic energy from Е F /2 to Е F is determined by the relation Similarly: The same result can be obtained from in a simpler form: With an accuracy of the order of unity, the number of states per unit energy interval near the Fermi energy is equal to the ratio of the number of conduction electrons to the Fermi energy. 1. Effective masses: germanium silicon those. in the valence band of germanium and silicon there are heavy and light holes. The valence bands consist of three subbands. 3. The diversity of properties of solids is evidence of the diversity of quasiparticles. 4. Until recently, it was believed that electrons are similar to each other. When one wants to emphasize the difference between iron electrons and copper electrons, one says that they have different Fermi surfaces. At the World Exhibition in Brussels, the building pays tribute to the age of physics. Represents the correct system of interconnected spheres, within which there are exhibition spaces. Each of which (sphere) represents an iron ion that has lost one electron. This is the Fermi level surface. Each metal has only its own inherent shape of the Fermi surface; it limits the region of momentum space occupied by conduction electrons at absolute zero. These are business cards of various metals. 5. The properties of metals are determined by electrons at or near the Fermi surface. 6. The motion of the wave packet associated with the wave vector is described by the equation group speed § Energy energy spectrum for free electrons in a periodic field The shaded areas of forbidden energy values (energy gaps) are shown in the figure. The wave function has the form: The energy is no longer a continuous function of the quasi-momentum, it is continuous only in the allowed energy zones and undergoes discontinuities at the boundaries of the Brillouin zones. Energy bands are a consequence of the periodic structure of a crystal and represent the fundamental characteristics of the electronic structure of a solid. is the zone boundary, this is the reciprocal lattice vector. The ranges of values at which the electron energy changes continuously, and undergoes a discontinuity at the boundaries, are called Brillouin zones. The energy spectrum of electrons and holes in the coordinates E - K. In germanium and silicon, the conduction band is described by two mass values. § Mechanism of electrical conductivity of intrinsic semiconductor The band with the highest energy containing electrons is called the valence band. The first zone with unoccupied energy levels is called the conduction band, since the electrons in this zone are involved in charge transfer. In conductors, the valence and conduction bands either coincide or overlap. In insulators and semiconductors, these zones are separated from each other. If the material is not in the ground state, but has additional energy - thermal excitation. This energy plays an important role in the electrical conductivity properties. The conductor is in the ground state if there is no thermal energy i.e. T = 0. The dependence of the probability of filling the energy levels with electrons at RT = 0 on the energy e is counted from the bottom of the band. for all energy values corresponding to filled levels. The energy measured from the bottom of the band, at which the value of f(E) changes abruptly from 1 to 0, is called the Fermi energy e F In this case, i.e. work output In the presence of thermal energy, some electrons will be excited and move from their original states to free energy levels. For electrons with energies near e F, such transitions are more probable, since a lower excitation energy is required. Correspondingly, the probability of filling the states also decreases with the growth of their energy. If the electrons do not obey the Pauli principle, then their energy distribution is described by the classical Maxwell-Boltzmann distribution The Fermi-Dirac distribution for various values of CT is shown in the figure. Here the Fermi energy has the meaning of the energy of the level corresponding to the 50% probability of filling. The number of free levels (vacancies) below the Fermi level, and their distribution with respect to e F coincides with the number and distribution of occupied states above the Fermi level. These states correspond to the thermal excitation of the electronic system and provide the appearance of the kinetic energy of directed motion. As the temperature rises (an increase in RT), the slope of the f(e) curve near e F decreases and the probability of filling high-energy states increases. It can be seen from the expressions for f(E, K, T) that the conductivity of materials strongly depends on temperature. In semiconductors, the position of the Fermi level formally corresponds to the top of the valence band, but this is not true. Let from the top of the valence band (with energy e V) an individual electron from excitation pass to the bottom (with energy e C) of the empty conduction band. e V is the ceiling of the valence band In the figure, the Fermi level is in the middle of the band gap, given the symmetry of the Fermi-Dirac distribution with respect to the Fermi energy e F and the apparent symmetry of the function f(E) in the gap between the top of the valence band and the bottom of the conduction band. * Let us determine the probability of an electron transition to the conduction band for diamond, the band gap e g »5.5 eV. at room temperature RT = 0.026 eV. for the bottom of the conduction band Thus, it is unlikely that even one out of every 10 44 electrons in the valence band will have enough energy to enter the conduction band at room temperature. Since each mole of a substance contains about 10 24 atoms. Therefore, diamond is a good insulator. Define for In this case, approximately one valence electron in a million can, upon excitation, go to the bottom of the conduction band, and electrons can be found in the conduction band. They will be much less than in the case of a conductor whose f(e) in the conduction band is on the order of unity. However, there are still enough electrons in the conduction band of the semiconductor to contribute to the electrical conductivity of the semiconductor. In semiconductors, f(e) strongly depends on temperature. An increase in temperature by 10 0 K relative to room temperature (300 0 K), i.e. by only 3%, the probability of electron transition to the conduction band increases by approximately 30%. As the band gap decreases, the temperature sensitivity of semiconductors increases. Excited with a transition to the conduction band, electrons leave behind unoccupied states or "holes" in the valence band. The initially filled valence band becomes partially filled and, consequently, energy excitations of electrons are possible in it, although a very small number. The hole behaves like a positively charged particle that can participate in electrical conduction. The real movement of electrons corresponds to a more or less free fictitious movement of holes in the direction of an external electric field. Holes react to an external force (for example, to an external electric field) differently than free electrons, therefore, in order to take into account the influence of other atoms on the mobility of holes, they are assigned an effective mass m *, which is slightly larger than the effective mass of an electron. Current Density of Electrons and Holes where n is the electron concentration, p is the concentration of holes, m n is the electron mobility, m p is the hole mobility. Under the action of an external electric field, electrons and holes acquire velocities of directed motion, drift velocities m n and m dr - mobilities For intrinsic semiconductors n=p Where n - strongly depends on the temperature in the conduction band, while the mobilities weakly depend on temperature If the electron concentration in the conduction band is low, then the probability of filling each level is small compared to unity in the denominator, then it can be neglected. The electrical conductivity of intrinsic semiconductors increases with temperature, while that of conductors decreases. If we take the logarithm This makes it possible, by measuring the electrical conductivity of a semiconductor at various temperatures, to experimentally determine the band gap for a given semiconductor. For metals, resistance increases with increasing temperature. R 0 -resistance at t \u003d 0 0 С R t - resistance at t 0 С a - thermal coefficient of resistance, equal to 1/273 For metals For semiconductors, resistance decreases rapidly with increasing temperature. where E a is the activation energy, it is different for different temperature ranges. The presence of an activation energy E a means that in order to increase the conductivity, it is necessary to supply energy to a semiconductor substance. Semiconductors are substances whose conductivity strongly depends on external conditions: temperature, pressure, external fields, irradiation with nuclear particles. Semiconductors are substances that have electrical conductivity at room temperature in the range from 10 -8 to 10 6 Sim m -1, which depends strongly on the type and amount of impurities, and the structure of the substance, and on external conditions. * In a semiconductor with intrinsic conductivity, the number of electrons is equal to the number of holes, each electron creates a single hole. The number of excited intrinsic carriers depends exponentially on , where E g is the energy band gap. If m C =m h , then i.e. the Fermi level lies in the middle of the bandgap. Index I (intrinsic - property) Does not contain the Fermi level. This is the law of mass action, which states that the distance of the Fermi level from the edges of both bands must be large compared to KT = 0.026 eV. At 300 0 K (room temperature), provided m e = m h = m, the product n i P i for germanium 3.6 × 10 27 cm -6 , for silicon 4.6 × 1019 cm -6 . The activation energy E a for an intrinsic semiconductor is equal to half the band gap Impurity semiconductors Arrangement of charges in the silicon lattice. Four A s electrons form tetrahedral covalent bonds similar to Si bonds, and the fifth A s electron conducts. Arsenic (As) has five valence electrons, while silicon (Si) has only four. The arsenic atom is called a donor, it donates an electron to the conduction band during ionization. The addition of an impurity to a semiconductor is called doping. E d = 0.020 ev., ionization energy At K W T<< E d
(низкая концентрация электронов проводимости) N d - donor concentration If a boron atom (B), which has three valence electrons, is introduced into silicon, it can “complete” its tetrahedral bonds by only borrowing one electron from the Si-Si bond, forming a hole in the silicon valence band, which takes part in conduction. The boron atom is called an acceptor precisely because it captures an electron from the valence band during ionization. Impurities that are not capable of ionization do not affect the concentration of carriers and may be present in large quantities - electrical measurements do not detect them. N a is the concentration of acceptors. The condition for the applicability of classical statistics is the inequality If the Fermi level lies above Ec by more than 5KT, then the semiconductor is completely degenerate. The degeneracy condition depends on the temperature and the position of the Fermi level relative to the bottom of the conduction band. Electron concentration in a non-degenerate semiconductor: F< E c
–KT, N c is the number of states in the conduction band The Fermi level is in the conduction band above its bottom by at least 5 kt. In a nondegenerate semiconductor, the hole concentration is determined by the Boltzmann statistics under the condition F > E v + KT i.e. the Fermi level lies above the top of the valence band by the value of CT. In a fully degenerate semiconductor, or F those. in the valence band below its ceiling by at least 5KT. N v is the number of states in the valence band. non-degenerate semiconductor Degenerate semiconductor In non-degenerate: In degenerate Where V F is the volume of the Brillouin zone. For spherical surfaces Electron distribution function: where g i is the degree of degeneracy, if E i =E d belongs to the donor impurity, then g i =2. If E i =E a belongs to the acceptor impurity, then g i =1/2 Distribution of electrons over donor levels by acceptor For holes: Number of electrons: Number of holes: N D \u003d N a \u003d 0 intrinsic semiconductor. Electroneutrality equation n = P. If N v = N c i.e. , Then Generation of conduction electrons and holes in intrinsic semiconductor: Temperature dependence of the Fermi level in an intrinsic semiconductor. As the temperature increases, the Fermi level approaches the band that has a lower density of states and therefore fills up faster. In the figure, the lnn i plot against the reciprocal temperature is a straight line: The dependence ln1/T compared to the linear term can be neglected. The angle of inclination of the straight line is determined by the width of the forbidden zone: Let us estimate the intrinsic concentration of charge carriers in germanium and silicon The concentration of charge carriers at T ® 0 vanishes, and the resistance of the intrinsic semiconductor must increase to infinity. However, in real semiconductors there always remains an impurity that provides conductivity at any temperature. Thermal generation in the figure of charge carriers in a semiconductor with a donor impurity. Low temperatures: conduction electrons are determined by the impurity concentration, which arises due to the ionization of the donor impurity. As the temperature rises, the Fermi level rises, passes through a maximum at a certain temperature, and then falls. When K d =N 2 C it is again in the middle between E C and E D . At a sufficiently high temperature N C >> N D , then the electron concentration does not depend on temperature and is equal to the impurity concentration. (Impurity depletion region). Charge carriers are called basic if their concentration is greater than the concentration of their own charge carriers n i at a given temperature, if the concentration is less than n i , then they are called minority charge carriers. In the region of impurity depletion, the concentration of minority charge carriers should sharply increase with temperature The latter is valid as long as the hole concentration remains much lower than the electron concentration. As the temperature increases, the number of holes increases and can become comparable with the electron concentration From the equation P=N D or n=2N D The transition temperature to its own concentration, the higher, the greater and the greater the concentration of impurities. acceptor semiconductor. Temperature dependence in the figure of the Fermi level in a semiconductor with an acceptor impurity. Let us estimate the temperature at which impurity depletion occurs. When all the impurity is ionized: When the entire impurity is ionized and the main substance is ionized: n=N D +P The wider the band gap and the higher the impurity concentration, the higher the temperature at which the transition to intrinsic conduction occurs. The band gap can be determined using the phenomenon of the internal photoelectric effect. If a semiconductor is irradiated with monochromatic light, gradually increasing the frequency of the light wave n, then, starting from a certain frequency, n 0, an increase in electrical conductivity (photoconductivity) can be detected. This frequency corresponds to such a photon energy hn 0 at which an electron in the main band, having absorbed a photon, receives energy from it sufficient to pass into the conduction band. This takes place if the inequality By measuring the frequency of light at which the increase in electrical conductivity begins, one can obtain . They get good results. Hall effect in a semiconductor. Physical phenomena that occur in a substance in a magnetic field when passing through a substance electric current under the influence of an electric field, called galvanomagnetic effects. In other words, galvanomagnetic phenomena are observed in matter under the combined action of electric and magnetic fields. Galvanomagnetic phenomena include: 1) Hall effect; 2) magnetoresistive effect, or magnetoresistance; 3) the Ettingshausen effect, or the transverse galvanothermomagnetic effect; 4) Nernot effect, or longitudinal galvanothermomagnetic effect. The Hall effect is also called the galvanomagnetic effect. The above names "transverse" and "longitudinal" galvanothermomagnetic effects reflect the direction of the temperature gradients relative to the current; with respect to the magnetic field, they can be transverse or longitudinal. Galvanomagnetic effects can be represented based on the consideration of the motion of a charged particle in electric and magnetic fields under the action of the Lorentz force: In parallel electric and magnetic fields, the particle moves along a helix with a continuously increasing step. A particle with a velocity V parallel along the field and V perp perpendicular to the field in one magnetic field rotates along a circle of radius with angular velocity and moves along the field at a speed of V parallel Since the electric field does not affect V perp, but changes the V param, it becomes obvious that the movement occurs along a helix with a variable pitch. In transverse (or crossed) fields, a particle that does not have an initial velocity moves along a cycloid: the particle rotates around a circle of radius (3) whose center moves uniformly in the direction perpendicular to the electric and magnetic fields with a drift velocity If the particle has an initial velocity V 0 lying in a plane perpendicular to the magnetic field, then the trajectory of the particle is a trachoid (an elongated or shortened cycloid). If the speed of a moving particle has a component along the magnetic field, then this velocity component is not affected by either the electric or magnetic fields. When a particle moves in a solid body, it is necessary to take into account collisions that disrupt the directed motion of particles under the action of fields. After each collision, the particle will move along a helix or tracheid, which is characterized by new parameters. To characterize the magnitude of the field, it is necessary to compare the relaxation time with the period of rotation of the particle under the action of a magnetic field. If the relaxation time significantly exceeds the period , then in time t the particle will make several revolutions, moving along a cycloid or a helix. This is possible in high magnetic fields. If the particle does not make even one revolution in time t, then magnetic fields are considered small. Thus, in strong fields in weak fields The concept of "strong" fields or "weak" depends not only on the magnitude of the magnetic field B, but also on the mobility of charge carriers. Conditions (5) and (6) can be related to the radius r of the circle along which the particle moves and the mean free path l: Therefore, in any magnetic fields r >> 1, the particle trajectory is slightly curved; in strong magnetic fields, the trajectory changes very strongly. To understand some phenomena, it is sufficient to take into account only the drift velocity while to understand other effects, it is important to keep in mind the spread of electron velocities. All this is taken into account by the kinetic equation, so it allows you to get a much more accurate description of the kinetic effects 1. Hall effect. The figure shows the appearance of the Hall field in electron and hole semiconductors. The semiconductor has the form of a parallelepiped with a section a × c, through which current flows. The electric field is directed along the X axis: magnetic field along the Y axis: When an electric field is turned on, an electric current is generated Carriers receive a speed of directed motion V d - drift speed - along the field for holes and against the field for electrons. When a magnetic field is turned on, a force acts on electrons and holes perpendicular and those. the Lorentz force does not depend on the sign of the charge carriers, but is determined only by the direction of the fields and , or and . It is pointing up in the picture. Charge carriers - electrons and holes - deviate in the same direction if their speed is determined by the electric field. As a result of the action of fields and collisions, electrons and holes will move along trajectories in the form of a straight line, averaging segments of the cycloids, at an angle j to the field. In other words, the vector will be rotated through an angle j relative to the vector, and the direction of rotation depends on the sign of the charge carriers, due to the fact that electrons and holes deviate in the same direction (in the figure, a, b). Thus must proceed in an unrestricted substance. If the semiconductor has finite dimensions in the direction of the Z axis, then as a result of the fact that the component j z ¹ 0, there will be an accumulation of carriers on the upper (in the figure) side of the sample, their deficit will appear on the lower. opposite sides sample are charged, and there is a transverse with respect to the electric field. This field is called the Hall field, and the phenomenon of the appearance of a transverse field under the action of a magnetic field is called the Hall effect. The direction of the Hall field depends on the sign of the charge carriers, in this case it is directed upwards in the n-sample and downwards in the p-sample. Prior to the application of a magnetic field to the sample, the equipotential surfaces were planes perpendicular to the X axis, i.e. vector, the value of E n will increase until the transverse field compensates for the Lorentz force. After that, the charge carriers will move as if only under the influence of one field, and the trajectory of the charge carriers will again be a straight line along the X axis, thereby the vector will be directed along the field. but the total electric field will be rotated through a certain angle j relative to the X axis or (Fig. c, 2). Thus, in an unlimited semiconductor, the current vector rotates, and in a limited semiconductor, the electric field vector rotates, and in any case, an angle j appears between and (or ), called the Hall angle. The equipotential surfaces in a limited sample are rotated by an angle j relative to their initial position, therefore, at points lying in the same perpendicular plane, a potential difference appears. where E n is the Hall field strength, and c is the size of the sample in the direction perpendicular to and: V n is called the Hall potential difference. Hall experimentally found that E n is determined by the current density and magnetic field induction, as well as the properties of the sample. The properties of the sample are determined by a certain value R, called the Hall coefficient. The four quantities: and R are related by the empirical relation It is easy to find R, given that the Hall field must compensate for the Lorentz force: This implies: On the other hand, according to (12) Comparing (14) and (15), we obtain n is the concentration of charge carriers (electrons or holes). The Hall coefficient is inversely proportional to the concentration of charge carriers and its sign coincides with the sign of the charge carriers. By determining R, one can find the sign of the charge carriers or the type of conduction. The sign of R is determined by the sign, or V n, if the sign of V n is determined accordingly. Hall angle j can be determined: For given and, the Hall field is determined only by the mobility of charge carriers. Estimate R. Let n = 10 16 cm -3 , then The resistance in a magnetic field increases, since the Hall field compensates for the effect of the magnetic field only on average, as if all charge carriers were moving at the same speed. However, the speeds of electrons (and holes) are different; therefore, particles moving at speeds greater than average speed, the magnetic field is stronger than the Hall field. Conversely, slower particles are deflected by the prevailing Hall field. As a result of the particle velocity spread, the contribution of fast and slow charge carriers to the conductivity decreases, which leads to an increase in resistance, but to a much lesser extent than in unlimited semiconductors. Physical foundations electronics Band theory of conductivity of solids According to physics, all substances consist of atoms, and atoms consist of a positive nucleus and electrons revolving around it in different orbits. Electrons in outer orbit are called valence and form bonds between adjacent atoms. Distinguish valence bond when the electron revolves around its orbit, and covalent bond when valence electrons revolve in a common orbit between two neighboring atoms. Electrons that leave their orbit and move freely in matter are called free and are involved in the conduction of electricity. All substances in relation to electric current are divided into: conductors Semiconductors insulators In a solid crystalline body consisting of many atoms, the electric and magnetic fields of individual atoms influence each other, forming energy levels. To explain the distinctive features of insulators, conductors and semiconductors, the band theory is used, according to which electrons, rotating around their nucleus in different orbits, have different energies. Rice. 1.1 - Energy zones of the insulator (a), conductor (b) and semiconductor (c). According to the band theory, the difference between these substances is as follows: · In insulators, all valence electrons are in their orbits, i.e. in the valence and free bands, but there are no electrons in the conduction band. To move from the valence band to the conduction band, it is necessary to tell the electron external influenceΔE to overcome the band gap. · In conductors, the valence band and the conduction band overlap each other, and under normal atmospheric conditions there are many free electrons in the metal. Semiconductors, like insulators, also have a band gap, but its thickness is much smaller, therefore, even under normal atmospheric conditions, they have free electrons, but their number is small compared to metals. The energy level in which the valence electrons are located form valence band. The energy level in which the free electrons involved in the conduction are located form conduction band. The valence and conduction bands are separated by a band gap. Band gap: The electrical conductivity of substances is determined by the content of free electrons. In metals, 1 cm3 contains about 1022 e/cm3, and in semiconductors 109÷1010 e/cm3. The electrons that provide the conductivity of a solid are called the electrons of the conduction band, and the word "zone" means a set of closely spaced energy levels. In presenting quantum laws, we will explain (vol. III, § 60) a very important and general principle that determines the distribution of electrons over possible energy levels, the so-called Pauli principle. For now, we only note that according to this principle, all electrons belonging to the same system have different quantum states. At equilibrium, the system has the lowest energy. But the Pauli principle complicates matters. According to the Pauli principle, the presence of electrons in identical, indistinguishable from each other quantum states is impossible. Therefore, with a sufficient number of electrons, all energy states with a minimum energy (“lower energy levels”) that are admissible according to quantum laws turn out to be filled, as it were. Since these low-energy states are “occupied” by some electrons, then, according to the Pauli principle, which “prohibits” electrons from being in identical states, the rest of the electrons “have to” occupy still unoccupied levels with higher energy. When identical atoms are combined into one crystal, the energy state of the electrons begins to be affected by the interaction of atoms. As a result of this interaction, any the energy state of an electron is split into close states, each of which can contain only one electron. Thus, instead of separate energy levels in an atom - in a crystal, wide energy bands are formed, or, as they are called, zones, the number of levels in which is equal to the number of atoms in the crystal (Fig. 114). In any solid body, both in a dielectric and in a conductor, there are electrons that reside at the lowest energy levels and "fill" all these levels. Rice. 114. Energy states of electrons. On the right - in an isolated atom, on the left - in a semiconductor. Such electrons are called filled band electrons. They do not participate in either electrical or thermal conduction. If the set of possible quantum levels is completely filled with electrons (saturated with them in the sense of the Pauli principle), then such a system of electrons turns out to be, as it were, constrained, deprived of the ability to participate in the phenomenon of electric current. The electric field, acting on the electron, would have to inform it of additional speed and thereby "raise" it to a nearby higher energy level. But if all possible energy levels are already "occupied", then this cannot happen. Only those electrons that are at the upper energy levels can participate in the phenomenon of electric current, and, moreover, in such a zone where levels not filled with electrons are located above the levels filled with electrons. Of course, there are always higher energy levels that are not yet filled with electrons, but it may happen that they are separated from the zone of filled levels by a large energy jump. In this case, i.e., when the zone of unfilled levels is separated from the zone of filled levels by a large energy difference, the electric field, which is capable of imparting only a small additional energy to the electron, obviously cannot transfer an electron from the level occupied by it to some other level and, therefore, the body will not have electrical conductivity. From what has been said, it is clear that the energy state of electrons in conductors and non-conductors can be represented by a very rough diagram shown in Fig. 115. We would be somewhat closer to reality if we imagined a huge number of electrons and a huge number of energy levels. It should be taken into account that the distribution of energy levels is uneven and different for bodies of different nature. Rice. 115 indicates only the main difference between conductors of electricity and non-conductors. Rice. 115. Energy schemes of non-conductor and conductor. The presence of electrons in the unfilled zone - in the conduction band - makes the body a conductor of electricity. There are many such electrons in metals even at absolute zero temperature. They do not exist in dielectrics. In semiconductors, they are available in a limited number. Sufficiently intense heating leads to the transfer of electrons from the filled band to the conduction band. High quality insulators are characterized by a large energy difference between higher levels filled zone and lower levels of the unfilled zone. Therefore, significant electronic conductivity is found in them only at very high temperatures. For semiconductors, on the contrary, the close arrangement of the mentioned zones is characteristic (Fig. 116). Therefore, although at low temperatures they do not conduct electricity at all, but already at a slight increase in temperature, many electrons in the semiconductor jump into the unfilled zone and the semiconductor acquires electrical conductivity. Very remarkable is a special type of electrical conductivity, which manifests itself due to the participation in the phenomenon of the electric current of the electrons of the filled zone, when this zone, due to the jump from it to the upper zone of some electrons, becomes partially empty (as can be seen, for example, from Fig. 116). The “free places” that have arisen at some levels are filled with electrons from the underlying levels under the action of an electric field. Newly formed free places are also filled with electrons that had even lower energy and received additional energy in the electric field. Thus, the “free space” (in other words, the “hole”) moves in the opposite direction to the movement of electrons. The hole moves like a positive charge. But this motion of the hole is actually only a manifestation of the displacement of a number of electrons under the action of the field. Rice. 116. Comparison of energy schemes of a good insulator and a semiconductor. Something similar can sometimes be observed in the lecture hall, where empty seats were found in the front rows. Listeners from the next rows move closer to the lecturer, and those who are even further away take their places. This is how empty seats move away from the lecturer, thus revealing the movement of listeners closer to the lecturer. The electrical conductivity of semiconductors is composed of electronic conductivity and hole conductivity. The electrical properties of semiconductors depend to a large extent on the presence of impurities. The influence of impurities can make the electrical conductivity of a semiconductor predominantly electronic or, conversely, predominantly hole. Together with additional atoms and electrons, impurities introduce intermediate energy levels between the filled band and the conduction band. On fig. 117 shows the energy scheme of a semiconductor with an admixture of atoms? which tells the semiconductor predominantly electronic conductivity (such impurities are called donors). In this case, the intermediate levels created by the impurity and filled with electrons are located close to the conduction band. Rice. 117. Influence of a donor on the energy scheme of electronic levels in a semiconductor. As the temperature rises, electrons from the intermediate levels created by the impurity can more easily jump into the conduction band than electrons from the filled band. Rice. 118. influence of an acceptor on the energy scheme of electronic levels in a semiconductor. Despite the occurrence of electronic conductivity, "free places" in the main filled zone may not be formed; hole conduction may be absent. An impurity of other atoms can impart predominantly hole conductivity to the semiconductor (such impurities are called acceptors). An excess of these atoms leads to the appearance intermediate levels not occupied by electrons and located close to the filled zone (Fig. 118). As the temperature rises, the electrons from the filled band jump to these intermediate levels, and a big number holes, which ensures electrical conductivity, despite the absence of electrons in the conduction band. For a better understanding of the nature of the conductivity created by an impurity, let us consider in more detail the action that is performed! impurity atom in the crystal lattice of a typical semiconductor - germanium. Germanium is a tetravalent element of the fourth group of the periodic system of Mendeleev. In the crystal lattice of germanium, each atom interacts with four nearest, neighboring atoms; eight electrons participate in this interaction: four electrons from the outer shell of the atom and four electrons from the outer shells of neighboring atoms (Fig. 119). Rice. 119. Electronic bonds in crystal lattices: a - pure germanium; b - in the presence of boron impurities; c - in the presence of phosphorus impurities. Let us assume that an extraneous atom with a different valence gets into the place of one of the germanium atoms. Then the system of valence bonds near the impurity atom will be broken. When this happens, one of two things happens: 1) if the impurity atom is a representative of the fifth group, i.e. pentavalent (for example, an atom or the fifth valence electron of the impurity atom, which turns out to be superfluous, easily separates from it and wanders around the crystal; in the presence of an applied electric field, this electron becomes a conduction electron, i.e., such an impurity turns out to be a donor (Fig. 117); 2) if an impurity atom in the germanium lattice is a representative of the third group (boron, aluminum or indium), i.e., trivalent, then such an atom is able to attach one electron to itself, borrowing it from a neighboring germanium atom, which requires the expenditure of some energy, communicated by thermal motion or photons. In this case, a vacant electronic site (“hole”) is formed in the germanium lattice. This vacancy does not remain permanently at any site, but due to electron transitions to this is a vacant place; it wanders chaotically around the crystal. In an electric field, the motion of a hole acquires a direction: during transitions, electrons will predominantly shift against the field, while the hole itself will move along the field, like a positive charge carrier (the relay motion of electrons is reduced to the motion of a hole). Semiconductors with predominantly electronic conductivity are called n-type semiconductors (negativ - negative), and semiconductors with hole conductivity are called p-type (positiv - positive).2.2.3 Phase response

Fig.2-5 Waveforms of input and output signals
Fig. 2-6 - Amplifier phase response2.2.4 Transient response.

Figure 2-7 Amplifier RH2.2.5 Typical amplifying stage on a transistor connected according to the OE circuit.

Rice. 2-8 Common Emitter Transistor Amplifier Stage
Fig.2-9 Types of Feedback
Fig.2-10 Serial voltage feedback2.3.2 Feedback in amplifiers

Fig.2-11 Emitter follower
Fig.2-12 Two stage amplifier
Fig.2-13 Amplifier equivalent circuit2.3.3 Thermal stabilization of the transistor amplifier
2.4 DC amplifiers
2.4.1 UPT on transistors.

Fig.2-14 Unbalanced DC
Fig.2-15 Balanced UPT2.4.2 Operational amplifiers

Fig. 2-16 Schematic designation of the op-amp2.4.2.1 Ways to turn on the OS

Fig. 217 Op-amp inverse connection
The amplifier is called inverting, since the output voltage is out of phase (inverse) with respect to the input. This is also indicated by the minus sign in formula (4).
Fig.2-18 Non-inverting op amp2.4.2.2 Adjusting the op amp gain

Fig.2-19 Gain adjustment by switch
Fig.2-20 Gain adjustment by potentiometer2.4.2.3 Op-amp selective amplifiers

Fig.2-21 Selective amplifier frequency response
Figure 2.22 Wien's bridge and its frequency response
Fig. 2-24 Op-amp selective amplifier
Fig.2-25 2T bridge and its frequency response2.4.2.4 Some applications of the DT

Fig.2-26 Current to voltage converter
Fig. 2-27 Voltage to current converter: loads R n
Fig.2-28 Op-amp voltage regulator
Fig.2-29 Stabilizer output with overload protection
Fig. 2-30 Op-amp power supply from two sources Fig 2-31 Scheme with a divider on zener diodes.
Fig. 2-32 Circuit with resistor divider3 Rectifiers
3.1 General theory

Fig3-1 Block diagram of small power rectifier3.2 Half-wave rectifier.

Fig.3-2 Half-wave rectifier3.3 Full wave rectifier

Figure 3-3 Full Wave Rectifiers3.4 Filters

Figure 3-4 Rectifier Equivalent Diagram
Fig.3-5 Half-wave rectifier
Fig.3-6 L-shaped LC filter
Fig.3-7 L-shaped RC filter4 Stabilizers
4.1 Parametric stabilizers

Fig.3-8 Rectifier load characteristics
Fig.3-9 The simplest stabilizer and its load characteristic. So that the current through the zener diode does not exceed I m ah, the resistor R b is turned on. When the load current or voltage changes, U f \u003d U b + U st changes, only U b, and U st \u003d U n remains constant.4.2 Compensating stabilizers

Fig.3-10 Compensation stabilizer
Fig.3-11. External characteristics of rectifiers:4.3 Switching voltage regulator

Fig.3-12 Switching voltage regulator5 Generators
5.1 Sine wave generator

Fig.3-13 LC generator with inductive feedback
Fig.1

Fig.2  ;
; 
 .
.![]() (3)
(3) is the area of the tooth.
is the area of the tooth.

Fig.3

Fig.4 
Fig.5 Fig.6 The spatial extent of electronic wave functions depends on quantum numbers. For large quantum numbers, the electronic wave functions extend to large distances from the nucleus, for these levels mutual influence atoms will appear at large distances between atoms. This is clearly seen in Fig. 7, using the levels of sodium atoms as an example. At the 1S, 2S, 2P levels, the influence of neighboring atoms is practically unaffected, while for the 3S, 3P and higher levels this influence is significant and these levels turn into energy bands. For 3S - electrons there is an energy minimum that provides a stable solid state configuration of sodium atoms at an average interatomic distance R ~ 3A. In the sodium atom, the energy of the 3S electron is affected by the influence of neighboring atoms, which also means a noticeable overlap of the wave functions of these electrons. Therefore, it is no longer possible to say that a specific 3S - electron is associated with a specific atom. When the presence of other atoms changes the potential well of an individual atom (Fig. 5, Fig. 6), the resulting Coulomb potential will no longer hold 3S - electrons around specific atoms, so that they can be located anywhere in the solid as a result of the overlap of 3S wave functions - electrons. But 3S - electrons cannot freely leave the solid, since their wave functions do not "go beyond" the substance. The binding energy of electrons in a solid is equal to the work function φ.
The spatial extent of electronic wave functions depends on quantum numbers. For large quantum numbers, the electronic wave functions extend to large distances from the nucleus, for these levels mutual influence atoms will appear at large distances between atoms. This is clearly seen in Fig. 7, using the levels of sodium atoms as an example. At the 1S, 2S, 2P levels, the influence of neighboring atoms is practically unaffected, while for the 3S, 3P and higher levels this influence is significant and these levels turn into energy bands. For 3S - electrons there is an energy minimum that provides a stable solid state configuration of sodium atoms at an average interatomic distance R ~ 3A. In the sodium atom, the energy of the 3S electron is affected by the influence of neighboring atoms, which also means a noticeable overlap of the wave functions of these electrons. Therefore, it is no longer possible to say that a specific 3S - electron is associated with a specific atom. When the presence of other atoms changes the potential well of an individual atom (Fig. 5, Fig. 6), the resulting Coulomb potential will no longer hold 3S - electrons around specific atoms, so that they can be located anywhere in the solid as a result of the overlap of 3S wave functions - electrons. But 3S - electrons cannot freely leave the solid, since their wave functions do not "go beyond" the substance. The binding energy of electrons in a solid is equal to the work function φ.
Fig.8

Fig.9 
Fig.11

![]()

Fig.13![]() (1)
(1)![]() (2)
(2)![]() (3)
(3)![]() (4)
(4) (5)
(5) (7)
(7) , (7)
, (7) ![]()


![]() etc.
etc.![]() ,
,

![]()
![]() (size of an atom),
(size of an atom), Where
Where
 E(K) is the quasi-momentum function. The energy of an electron in an ideal lattice is a periodic function of the quasi-momentum.
E(K) is the quasi-momentum function. The energy of an electron in an ideal lattice is a periodic function of the quasi-momentum.
Electron -
Photon electromagnetic wave
Phonon elastic wave
Plasmon Collective electron wave
magnon remagnetization wave
---
Polaron Electron + elastic deformation
---
exciton polarization wave

![]()

 In the ground state (1S) of a system of N free electrons, the occupied states can be described by points inside the sphere in K-space. The energy corresponding to the surface of this sphere is the Fermi energy. The wave vectors "resting" against the surface of this sphere have lengths equal to K F , and the surface itself is called the Fermi surface (in this state it is a sphere). K F - radius of this sphere
In the ground state (1S) of a system of N free electrons, the occupied states can be described by points inside the sphere in K-space. The energy corresponding to the surface of this sphere is the Fermi energy. The wave vectors "resting" against the surface of this sphere have lengths equal to K F , and the surface itself is called the Fermi surface (in this state it is a sphere). K F - radius of this sphere






 its value can be calculated at T=0 by the formula
its value can be calculated at T=0 by the formula 
![]() , this is the degeneracy condition, the distribution function of such particles is close to the "step"
, this is the degeneracy condition, the distribution function of such particles is close to the "step"

![]()
![]()
 dimensionless parameter
dimensionless parameter![]() .
.
 ;
; 






![]()









 2. The Fermi surface is the surface of constant energy in space. Fermi surface at absolute zero separates electron-filled states from unfilled states. Fermi sphere. All states with K<К F являются занятыми.
2. The Fermi surface is the surface of constant energy in space. Fermi surface at absolute zero separates electron-filled states from unfilled states. Fermi sphere. All states with K<К F являются занятыми.





Fig.1 
Fig.2  ;
; 
 .
.![]() (3)
(3) is the area of the tooth.
is the area of the tooth.
Fig.4 
Fig.5 Fig.6 The spatial extent of electronic wave functions depends on quantum numbers. For large quantum numbers, the electronic wave functions extend to large distances from the nucleus; for these levels, the mutual influence of atoms will manifest itself at large distances between atoms. This is clearly seen in Fig. 7, using the levels of sodium atoms as an example. At the 1S, 2S, 2P levels, the influence of neighboring atoms is practically unaffected, while for the 3S, 3P and higher levels this influence is significant and these levels turn into energy bands. For 3S - electrons there is an energy minimum that provides a stable solid state configuration of sodium atoms at an average interatomic distance R ~ 3A. In the sodium atom, the energy of the 3S electron is affected by the influence of neighboring atoms, which also means a noticeable overlap of the wave functions of these electrons. Therefore, it is no longer possible to say that a specific 3S - electron is associated with a specific atom. When the presence of other atoms changes the potential well of an individual atom (Fig. 5, Fig. 6), the resulting Coulomb potential will no longer hold 3S - electrons around specific atoms, so that they can be located anywhere in the solid as a result of the overlap of 3S wave functions - electrons. But 3S - electrons cannot freely leave the solid, since their wave functions do not "go beyond" the substance. The binding energy of electrons in a solid is equal to the work function φ.
The spatial extent of electronic wave functions depends on quantum numbers. For large quantum numbers, the electronic wave functions extend to large distances from the nucleus; for these levels, the mutual influence of atoms will manifest itself at large distances between atoms. This is clearly seen in Fig. 7, using the levels of sodium atoms as an example. At the 1S, 2S, 2P levels, the influence of neighboring atoms is practically unaffected, while for the 3S, 3P and higher levels this influence is significant and these levels turn into energy bands. For 3S - electrons there is an energy minimum that provides a stable solid state configuration of sodium atoms at an average interatomic distance R ~ 3A. In the sodium atom, the energy of the 3S electron is affected by the influence of neighboring atoms, which also means a noticeable overlap of the wave functions of these electrons. Therefore, it is no longer possible to say that a specific 3S - electron is associated with a specific atom. When the presence of other atoms changes the potential well of an individual atom (Fig. 5, Fig. 6), the resulting Coulomb potential will no longer hold 3S - electrons around specific atoms, so that they can be located anywhere in the solid as a result of the overlap of 3S wave functions - electrons. But 3S - electrons cannot freely leave the solid, since their wave functions do not "go beyond" the substance. The binding energy of electrons in a solid is equal to the work function φ.
Fig.8
Fig.9 
Fig.11
![]()

Fig.13![]() (1)
(1)![]() (2)
(2)![]() (3)
(3)![]() (4)
(4) (5)
(5) (7)
(7) , (7)
, (7) ![]()


![]() etc.
etc.![]() ,
,

![]()
![]() (size of an atom),
(size of an atom),Law of motion compared to
 Where
Where
 E(K) is the quasi-momentum function. The energy of an electron in an ideal lattice is a periodic function of the quasi-momentum.
E(K) is the quasi-momentum function. The energy of an electron in an ideal lattice is a periodic function of the quasi-momentum.
![]()

 In the ground state (1S) of a system of N free electrons, the occupied states can be described by points inside the sphere in K-space. The energy corresponding to the surface of this sphere is the Fermi energy. The wave vectors "resting" against the surface of this sphere have lengths equal to K F , and the surface itself is called the Fermi surface (in this state it is a sphere). K F - radius of this sphere
In the ground state (1S) of a system of N free electrons, the occupied states can be described by points inside the sphere in K-space. The energy corresponding to the surface of this sphere is the Fermi energy. The wave vectors "resting" against the surface of this sphere have lengths equal to K F , and the surface itself is called the Fermi surface (in this state it is a sphere). K F - radius of this sphere






 its value can be calculated at T=0 by the formula
its value can be calculated at T=0 by the formula 
![]() , this is the degeneracy condition, the distribution function of such particles is close to the "step"
, this is the degeneracy condition, the distribution function of such particles is close to the "step"

![]()
![]()
 dimensionless parameter
dimensionless parameter![]() .
.
 ;
; 






![]()




conclusions





 2. The Fermi surface is the surface of constant energy in space. The Fermi surface at absolute zero separates the electron-filled states from the unfilled states. Fermi sphere. All states with K<К F
являются занятыми.
2. The Fermi surface is the surface of constant energy in space. The Fermi surface at absolute zero separates the electron-filled states from the unfilled states. Fermi sphere. All states with K<К F
являются занятыми.![]()


![]()
![]()





 The distribution that takes into account the Pauli principle is called the Fermi–Dirac distribution
The distribution that takes into account the Pauli principle is called the Fermi–Dirac distribution


e C is the bottom of the conduction band. ![]() probability at RT = 0.026 eV. (room)
probability at RT = 0.026 eV. (room)
![]()
![]()
![]()
![]() , s - coefficient
, s - coefficient
 and therefore
and therefore ![]() , or
, or ![]()

![]()
 and build a graph of lns versus , then we get a straight line, the slope of which is equal to
and build a graph of lns versus , then we get a straight line, the slope of which is equal to

![]()
![]()
![]() or where KV \u003d E a, then
or where KV \u003d E a, then ![]()







 Where
Where 


![]() , whence E F
, whence E F 

Degenerate semiconductor
 it does not depend on temperature.
it does not depend on temperature.![]()


![]()
![]()
![]() does not depend on the Fermi level
does not depend on the Fermi level![]() , where the radius of the Fermi sphere
, where the radius of the Fermi sphere![]()



 ;
; 




![]() where
where ![]() the position of the Fermi level does not depend on temperature and lies in the middle of the band gap. The intrinsic semiconductor is non-degenerate.
the position of the Fermi level does not depend on temperature and lies in the middle of the band gap. The intrinsic semiconductor is non-degenerate.![]() or
or 
![]()
![]() where
where ![]() tgs is measured according to the schedule (lnn i , 1/T)
tgs is measured according to the schedule (lnn i , 1/T) are equal to 0.299 and 0.719, and at T»300 0 K,
are equal to 0.299 and 0.719, and at T»300 0 K,![]() And
And ![]()

![]()




High temperatures
 the entire impurity is ionized and it is necessary to take into account the ionization of the substance.
the entire impurity is ionized and it is necessary to take into account the ionization of the substance.![]()



Photoconductivity
![]()
![]() (1)
(1) (2)
(2) (5)
(5) (6)
(6)
![]() (9)
(9) (10)
(10) (11)
(11)![]() (12)
(12)![]() (13)
(13)![]() (15)
(15)![]()


Germanium (Ge) 0.85 eV;
Silicon (Si) 1.1 eV;
Indium phosphide (JnP) 1.26 eV;
Metals (Cu) 0 eV;
Insulators >3 eV.
To create a current I=1A must be skipped ne≈1018 per second.