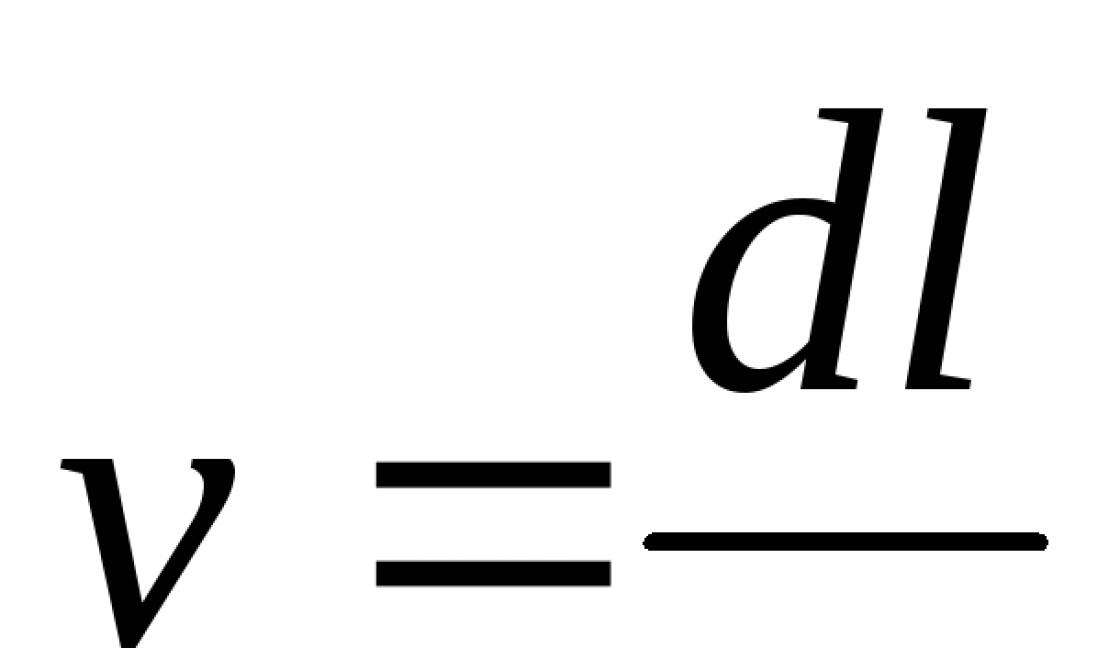Hydrochloric acid - (hydrochloric acid, an aqueous solution of hydrogen chloride), known as the formula HCl - caustic chemical compound. Since ancient times, people have used this colorless liquid for various purposes, emitting a light smoke in the open air.
Properties of a chemical compound
HCl is used in various areas human activity. It dissolves metals and their oxides, is absorbed in benzene, ether and water, does not destroy fluoroplastic, glass, ceramics and graphite. Its safe use is possible when stored and operated under the correct conditions, with all safety precautions observed.
Chemically pure (chemically pure) hydrochloric acid is formed during gaseous synthesis from chlorine and hydrogen, giving hydrogen chloride. It is absorbed in water, obtaining a solution with an HCl content of 38-39% at +18 C. An aqueous solution of hydrogen chloride is used in various fields of human activity. The price of chemically pure hydrochloric acid is variable, and depends on many components.
Scope of application of an aqueous solution of hydrogen chloride
Usage of hydrochloric acid gained popularity due to its chemical and physical properties:
- in metallurgy, in the production of manganese, iron and zinc, in technological processes, in metal refining;
- in galvanoplasty - during etching and pickling;
- in the production of soda water to regulate acidity, in the manufacture of alcoholic beverages and syrups in the food industry;
- for leather processing in light industry;
- when treating non-potable water;
- for optimization of oil wells in the oil industry;
- in radio engineering and electronics.
Hydrochloric acid (HCl) in medicine
The most famous property of a hydrochloric acid solution is the alignment of the acid-base balance in the human body. A weak solution, or drugs, treats low acidity of the stomach. This optimizes the digestion of food, helps fight germs and bacteria that enter from the outside. Chemically pure hydrochloric acid helps to normalize the low level of gastric acidity and optimizes the digestion of proteins.
Oncology uses HCl to treat neoplasms and slow their progression. Hydrochloric acid preparations are prescribed for the prevention of stomach cancer, rheumatoid arthritis, diabetes, asthma, urticaria, cholelithiasis and others. In folk medicine, hemorrhoids are treated with a weak acid solution.
You can learn more about the properties and types of hydrochloric acid.
What is hydrochloric acid solution? It is a compound of water (H2O) and hydrogen chloride (HCl), which is a colorless thermal gas with a characteristic odor. Chlorides are highly soluble and decompose into ions. Hydrochloric acid is the most well-known compound that forms HCl, so we can talk about it and its features in detail.
Description
Hydrochloric acid solution belongs to the class of strong. It is colorless, transparent and caustic. Although technical hydrochloric acid has a yellowish color due to the presence of impurities and other elements. It "smokes" in the air.
It is worth noting that this substance is also present in the body of every person. In the stomach, to be more precise, at a concentration of 0.5%. Interestingly, this amount is enough to completely destroy the razor blade. The substance will corrode it in just a week.
Unlike the same sulfuric acid, by the way, the mass of hydrochloric acid in solution does not exceed 38%. We can say that this indicator is a “critical” point. If you start to increase the concentration, then the substance will simply evaporate, as a result of which hydrogen chloride will simply evaporate with water. Plus, this concentration is maintained only at 20 ° C. The higher the temperature, the faster the evaporation.
Interaction with metals
Hydrochloric acid solution can enter into many reactions. First of all, with metals that stand before hydrogen in a series of electrochemical potentials. This is the sequence in which the elements go as their characteristic measure, the electrochemical potential (φ 0), increases. This indicator is extremely important in the cation reduction half-reactions. In addition, it is this series that demonstrates the activity of metals, which they exhibit in redox reactions.
So, interaction with them occurs with the release of hydrogen in the form of gas and with the formation of salt. Here is an example of a reaction with sodium, mild alkali metal: 2Na + 2HCl → 2NaCl + H 2 .
With other substances, the interaction proceeds according to similar formulas. This is how the reaction with aluminum, a light metal, looks like: 2Al + 6HCl → 2AlCl 3 + 3H 2.

Reactions with oxides
Hydrochloric acid solution also interacts well with these substances. Oxides are binary compounds of an element with oxygen, having an oxidation state of -2. All known examples are sand, water, rust, dyes, carbon dioxide.
Hydrochloric acid does not interact with all compounds, but only with metal oxides. The reaction also produces a soluble salt and water. An example is the process between acid and magnesium oxide, alkaline earth metal: MgO + 2HCl → MgCl 2 + H 2 O.
Reactions with hydroxides
This is the name of inorganic compounds in the compositions of which there is a hydroxyl group -OH, in which the hydrogen and oxygen atoms are connected covalent bond. And, since the hydrochloric acid solution only interacts with metal hydroxides, it is worth mentioning that some of them are called alkalis.
So the resulting reaction is called neutralization. Its result is the formation of a weakly dissociating substance (i.e. water) and salt.
An example is the reaction of a small volume of hydrochloric acid solution and barium hydroxide, a soft alkaline earth malleable metal: Ba(OH) 2 + 2HCl = BaCl 2 + 2H 2 O.

Interaction with other substances
In addition to the above, hydrochloric acid can also react with other types of compounds. In particular with:
- Metal salts, which are formed by other, weaker acids. Here is an example of one of these reactions: Na 2 Co 3 + 2HCl → 2NaCl + H 2 O + CO 2. Shown here is the interaction with the salt formed carbonic acid(H 2 CO 3).
- Strong oxidizers. With manganese dioxide, for example. Or with potassium permanganate. These reactions are accompanied by the release of chlorine. Here is one example: 2KMnO 4 + 16HCl → 5Cl 2 + 2MnCl 2 + 2KCl + 8H 2 O.
- ammonia. It is hydrogen nitride with the formula NH 3 , which is a colorless but pungent gas. The consequence of its reaction with a solution of hydrochloric acid is a mass of thick white smoke, consisting of small crystals of ammonium chloride. Which, by the way, is known to everyone as ammonia (NH 4 Cl). The interaction formula is as follows: NH 3 + HCl → NH 4 CL.
- Silver nitrate - an inorganic compound (AgNO 3), which is a salt of nitric acid and silver metal. Due to the contact of a solution of hydrochloric acid with it, a qualitative reaction occurs - the formation of a cheesy precipitate of silver chloride. which does not dissolve in nitric acid. It looks like this: HCL + AgNO 3 → AgCl ↓ + HNO 3.

Getting a substance
Now we can talk about what they do to form hydrochloric acid.
First, by burning hydrogen in chlorine, the main component, gaseous hydrogen chloride, is obtained. which is then dissolved in water. The result of this simple reaction is the formation of a synthetic acid.
This substance can also be obtained from off-gases. These are chemical waste (side) gases. They are formed by a variety of processes. For example, when chlorinating hydrocarbons. The hydrogen chloride in their composition is called off-gas. And the acid thus obtained, respectively.
It should be noted that in last years the share of the off-gas substance in the total volume of its production is increasing. And the acid formed as a result of burning hydrogen in chlorine is displaced. However, in fairness, it should be noted that it contains fewer impurities.

Application in everyday life
Many cleaning products that householders use regularly contain a certain amount of hydrochloric acid solution. 2-3 percent, and sometimes less, but it's there. That is why, putting plumbing in order (washing tiles, for example), you need to wear gloves. Highly acid products can harm the skin.
Another solution is used as a stain remover. It helps to get rid of ink or rust on clothes. But in order for the effect to be noticeable, it is necessary to use a more concentrated substance. A 10% hydrochloric acid solution will do. He, by the way, perfectly removes scale.
It is important to store the substance correctly. Keep acid in glass containers and in places where animals and children cannot reach. Even a weak solution that gets on the skin or mucous membranes can cause a chemical burn. If this happens, immediately rinse the areas with water.
In the field of construction
The use of hydrochloric acid and its solutions is a popular way to improve many building processes. For example, it is often added to concrete mix to increase frost resistance. In addition, this way it hardens faster, and the resistance of the masonry to moisture increases.
Hydrochloric acid is also used as a limestone cleaner. Its 10% solution - The best way fighting dirt and marks on red brick. It is not recommended to use it for cleaning others. The structure of other bricks is more sensitive to the action of this substance.

In medicine
In this area, the substance under consideration is also actively used. Dilute hydrochloric acid has the following effects:
- Digests proteins in the stomach.
- Stops the development of malignant tumors.
- Helps in the treatment of cancer.
- Normalizes acid-base balance.
- Serves as an effective tool in the prevention of hepatitis, diabetes, psoriasis, eczema, rheumatoid arthritis, cholelithiasis, rosacea, asthma, urticaria and many other ailments.
Did you come up with the idea to dilute the acid and use it inside in this form, and not as part of medicines? This is practiced, but it is strictly forbidden to do this without medical advice and instructions. Having calculated the proportions incorrectly, you can swallow an excess of hydrochloric acid solution, and simply burn your stomach.
By the way, you can still take medications that stimulate the production of this substance. And not just chemicals. The same calamus, peppermint and wormwood contribute to this. You can make decoctions based on them yourself, and drink them for prevention.

Burns and poisoning
As effective as this remedy is, it is dangerous. Hydrochloric acid, depending on the concentration, can cause chemical burns of four degrees:
- There is only redness and pain.
- There are blisters with a clear liquid and swelling.
- Formed necrosis of the upper layers of the skin. Blisters fill with blood or cloudy contents.
- The lesion reaches the tendons and muscles.
If the substance somehow got into the eyes, it is necessary to rinse them with water, and then with a soda solution. But in any case, the first thing to do is to call an ambulance.
The ingestion of acid inside is fraught with acute pains in the chest and abdomen, swelling of the larynx, vomiting bloody masses. As a result, severe pathologies of the liver and kidneys.
And the first signs of poisoning in pairs include a dry frequent cough, choking, damage to the teeth, burning in the mucous membranes and abdominal pain. The first emergency aid is washing and rinsing the mouth with water, as well as access to fresh air. Only a toxicologist can provide real help.
1.2679; G crnt 51.4°C, p crit 8.258 MPa, d crit 0.42 g/cm 3 ; -92.31 kJ / mol, D H pl 1.9924 kJ / mol (-114.22 ° C), D H test 16.1421 kJ / mol (-8.05 ° C); 186.79 J / (mol ) TO); vapor pressure (Pa): 133.32 10 -6 (-200.7 ° C), 2.775 10 3 (-130.15 ° C), 10.0 10 4 (-85.1 ° C), 74.0 10 4 (-40 ° C), 24.95 10 5 (O ° C), 76.9 10 5 (50 ° C); the equation for the temperature dependence of vapor pressure lgp (kPa) = -905.53 / T + 1.75lgT- -500.77 10 -5 T + 3.78229 (160-260 K); coefficient compressibility 0.00787; g 23 mN/cm (-155°C); r 0.29 10 7 Ohm m (-85°C), 0.59 10 7 (-114.22°C). See also table. 1.

Solubility of HCl in hydrocarbons at 25 °C and 0.1 MPa (mol.%): in pentane-0.47, hexane-1.12, heptane-1.47, octane-1.63. The p-value of HC1 in alkyl and aryl halides is low, for example. 0.07 mol / mol for C 4 H 9 C1. P-value in the range from -20 to 60 ° C decreases in the series dichloroethane-tri-chloroethane-tetrachloroethane-trichlorethylene. P-value at 10 ° C in a number of alcohols is approximately 1 mol / mol of alcohol, in carboxylic esters to-t 0.6 mol / mol, in carboxylic acids 0.2 mol / mol. In simple ethers, stable adducts R 2 O HCl are formed. The p-value of HC1 in chloride melts obeys Henry's law and amounts to 2.51 10 -4 (800 ° C), 1.75 10 -4 mol / mol (900 ° C) for KCl, 1.90 10 for NaCl -4 mol / mol (900 ° C).
Salt to-ta. The dissolution of HCl in water is highly exothermic. process, for infinitely razb. water solution D H 0 dissolution of HCl -69.9 kJ / mol, Cl ion -- 167.080 kJ/mol; HC1 in water is completely ionized. The p-value of HC1 in water depends on the temperature (Table 2) and the partial pressure of HC1 in the gas mixture. Density of hydrochloric acid decomp. concentrations and h at 20 °C are presented in Table. 3 and 4. With an increase in the temperature h of hydrochloric acid decreases, for example: for 23.05% hydrochloric acid at 25 ° C h 1364 mPa s, at 35 ° C 1.170 mPa s. hydrochloric acid containing h moles water per 1 mole of HC1 is [kJ/(kg K)]: 3.136 (n = 10), 3.580 (n = 20), 3.902 (n = 50), 4.036 (n = 100), 4.061 (n = 200 ).




HCl forms an azeotropic mixture with water (Table 5). In the HCl-water system, there are three eutectic. points: - 74.7 ° C (23.0% by mass of HCl); -73.0°C (26.5% HCl); -87.5°C (24.8% HC1, metastable phase). Known crystalline hydrates Hcl nH 2 O, where n = 8.6 (mp. -40 ° C), 4. 3 (mp. -24.4 ° C), 2 (mp. -17, 7°C) and 1 (mp. -15.35°C). Ice crystallizes from 10% hydrochloric acid at -20, from 15% at -30, from 20% at -60 and from 24% at -80°C. The p-value of metal halides decreases with an increase in the concentration of HCl in hydrochloric acid, which is used to salt them out.
Chemical properties. Pure dry HCl begins to dissociate above 1500°C, it is chemically passive. Mn. metals, C, S, P do not interact. even with liquid HCl. It reacts with nitrides, carbides, borides, sulfides above 650 ° C, with hydrides Si, Ge and B-in are present. AlCl 3, with oxides of transition metals - at 300 ° C and above. O 2 and HNO 3 are oxidized to Cl 2, with SO 3 gives C1SO 3 H. O p-tions with org. compounds, see Hydrohalogenation.
WITH hydrochloric acid is chemically very active. Dissolves with the release of H 2 all metals having negative. normal potential,with me. metal oxides and hydroxides forms chlorides, releases free. to-you from such salts as phosphates, silicates, borates, etc.
Receipt. In the industry, Hcl get a trace. ways-sulfate, synthetic. and from off-gases (side gases) of a number of processes. The first two methods lose their meaning. So, in the USA in 1965 the share of waste hydrochloric acid was 77.6% in the total volume of production, and in 1982-94%.
The production of hydrochloric acid (reactive, obtained by the sulfate method, synthetic, off-gas) consists in obtaining HCl with the last. its absorption by water. Depending on the method of removing the heat of absorption (reaches 72.8 kJ / mol), the processes are divided into isothermal, adiabatic. and combined.
The sulfate method is based on the interaction. NaCl with conc. H 2 SO 4 at 500-550 ° C. reaction gases contain from 50-65% HCl (muffle furnaces) to 5% HCl (fluidized bed reactor). It is proposed to replace H 2 SO 4 with a mixture of SO 2 and O 2 (process temperature approx. 540 ° C, cat.-Fe 2 O 3).
The basis of the direct synthesis of HCl is the chain burning p-tion: H 2 + Cl 2 2HCl + 184.7 kJ. The equilibrium constant K p is calculated from the equation: lgK p \u003d 9554 / T- 0.5331g T + 2.42.
R-tion is initiated by light, moisture, porous solids (charcoal, porous Pt) and some minerals. in-you (quartz, clay). Synthesis is carried out with an excess of H 2 (5-10%) in combustion chambers made of steel, graphite, quartz, refractory bricks. Naib. modern HCl pollution prevention material - graphite impregnated with phenol-formald. resins. To prevent the explosive nature of combustion, the reagents are mixed directly in the flame of the burner. To the top. heat exchangers are installed in the zone of combustion chambers to cool the reaction. gases up to 150-160°С. The power of modern graphite furnaces reaches 65 tons / day (in terms of 35% hydrochloric acid). In case of H 2 deficiency, decomp. process modifications; for example, pass a mixture of Cl 2 with water vapor through a layer of porous hot coal:
2Cl 2 + 2H 2 O + C: 4HCl + CO 2 + 288.9 kJ
The temperature of the process (1000-1600 ° C) depends on the type of coal and the presence of impurities in it that are catalysts (eg, Fe 2 O 3). It is promising to use a mixture of CO with water vapor:
CO + H 2 O + Cl 2: 2HCl + CO 2
More than 90% of hydrochloric acid in developed countries is obtained from off-gas HCl, which is formed during chlorination and dehydrochlorination of org. compounds, pyrolysis chlororg. waste, metal chlorides, obtaining potassium non-chlorinated. fertilizers, etc. Abgases contain decomp. quantity of HC1, inert impurities (N 2, H 2, CH 4), slightly soluble in water org. in-va (chlorobenzene, chloromethanes), water-soluble in-va (acetic acid, chloral), acidic impurities (Cl 2, HF, O 2) and water. The use of isothermal absorption is advisable at a low content of HC1 in exhaust gases (but with a content of inert impurities less than 40%). Naib. film absorbers are promising, allowing to extract from 65 to 85% HCl from the initial off-gas.
Naib. adiabatic schemes are widely used. absorption. Abgases are introduced into the lower. part of the absorber, and water (or dilute hydrochloric acid) countercurrent to the top. Hydrochloric acid is heated to the boiling point due to the heat of dissolution of HCl. Change t-ry absorption and concentration of Hcl is given in fig. 1. The absorption temperature is determined by the boiling temperature of the corresponding concentration (max. boiling temperature of the azeotropic mixture is approx. 110 ° C).
On fig. 2 shows a typical adiabatic scheme. absorption of HCl from off-gases formed during chlorination (eg, obtaining chlorobenzene). HCl is absorbed in the absorber 1, and the remains of water-soluble org. in-in is separated from water after condensation in the apparatus 2, further purified in the tail column 4 and separators 3, 5 and commercial hydrochloric acid is obtained.

Rice. 1. Distribution scheme t-r (curve 1) and
).
In the absence of hydrometers, the density ρ (g / cm 3) is calculated from the mass m (g) of a known volume of acid V (cm 3) measured on an electronic balance: ρ = m/V.
It is convenient and safe to take acid into a polypropylene syringe with a 20 ml scale by smoothly moving the piston until it stops.
Volume V corresponds to the complete filling of the syringe. To determine this volume, place the dry syringe on the weighing pan and zero out the tare weight (or record the weight of the empty syringe). Fill the entire volume of the syringe with distilled water, avoiding air bubbles, carefully wipe the surface of the syringe and reweigh it.
Assuming the density of water ρw = 0.998 g/cm 3 (at 20 °C), determine the volume of the syringe
V = mv / 0.998, where mw is the mass of water (g).
Then completely fill the syringe with the available acid solution, measure the mass of the solution and calculate the density of the acid using the formula above. If the obtained density value is less than 1.174 g/cm 3 , the concentrated acid does not meet the requirements of GOST 3118-78 or is diluted with water.
Example.
The acid is taken into a syringe, the total volume of which is V = 24.6 cm 3 . The mass of acid, measured on electronic scales, m = 29.175 g.
Therefore, the calculated density value ρ \u003d 29.175 / 24.6 \u003d 1.186 g / cm 3.
2. Determination of the concentration of aqueous solutions of hydrochloric acid.
The concentration of hydrochloric acid solutions can be expressed as a percentage of HCL in the mass of the solution, as a volume ratio of shares concentrated acid and water in solution, as well as the number of moles of a substance in a liter of solution.The concentration of the solution is determined by density using the values given in the reference tables.
Example.
The mass of a hydrochloric acid solution with a volume of 24.6 cm 3 is 26.2 g. It is necessary to determine in what volume ratio the concentrated acid is mixed with water, the initial concentration, as well as the weight and molar concentration (normality) of the solution.
According to the calculated value of the density of the solution ρ \u003d 26.2 / 24.6 \u003d 1.065 g / cm 3 use table 3 to determine the volume fractions of HCL and water (1:2) and the initial concentration of the acid from which the solution was prepared (36.5% wt.).
Then, using table 4, find for a solution with a density of 1.065 g / cm 3 by interpolating the values \u200b\u200bof the molar concentration:
3.881 + (4.004 - 3.881) (36.5 - 36.0) = 3.942 mol / l
Then, using table 5, determine the weight concentration of the solution:
13.30 + (13.69 - 13.30) (36.5 - 36.0) = 13.49% wt.
3. Preparation of aqueous solutions of hydrochloric acid in a given volume ratio.
To prepare solutions, it is necessary to use hydrochloric acid according to GOST 3118-78 with a weight concentration of 35 to 38 wt%. (Table 1).If the concentration of the acid is not known, determine it from the density.
The preparation of the solution must be carried out by adding a volume of concentrated acid to a given volume of distilled water, observing the safety requirements. Use an appropriate container to prepare the solution. Work under a hood.
Example.
To prepare 500 ml of a solution in a 1:4 volume ratio, carefully pour 100 ml of concentrated acid into 400 ml of distilled water, mix thoroughly and pour the solution into a dark glass container with a sealed lid.
4. Preparation of aqueous solutions of hydrochloric acid of the required weight concentration.
To prepare the solution, it is necessary to mix the calculated amounts of an acid of a known concentration and distilled water.Example.
It is necessary to prepare 1 liter of HCL solution with a concentration of 6% by weight. from hydrochloric acid with a concentration of 36% wt. (such a solution is used in KM carbonate meters manufactured by OOO NPP Geosfera).
According to Table 2, determine the molar concentration of acid with a weight fraction of 6% wt. (1.692 mol / l) and 36% wt. (11.643 mol / l).
Calculate the volume of concentrated acid containing the same amount of HCl (1.692 g-eq.) as in the prepared solution:
1.692 / 11.643 = 0.1453 liters.
Therefore, by adding 145 ml of acid (36% by weight) to 853 ml of distilled water, you get a solution of a given weight concentration.
5. Preparation of aqueous solutions of hydrochloric acid of a given molar concentration.
To prepare a solution with the desired molar concentration (Mp), it is necessary to pour one volume of concentrated acid (V) into a volume (Vv) of distilled water, calculated by the ratioVv \u003d V (M / Mp - 1)
Where M is the molar concentration of the original acid.
If the acid concentration is not known, determine it from the density using Table 2.
Example.
The weight concentration of the acid used is 36.3% wt. It is necessary to prepare 1 l of an aqueous solution of HCL with a molar concentration of 2.35 mol/l.
From Table 1, interpolate the values 12.011 mol/l and 11.643 mol/l to find the molar concentration of the acid used:
11.643 + (12.011 - 11.643) (36.3 - 36.0) = 11.753 mol/l
Use the above formula to calculate the volume of water:
Vv \u003d V (11.753 / 2.35 - 1) \u003d 4 V
Taking Vv + V = 1 l, get the volume values: Vv = 0.2 l and V = 0.8 l.
Therefore, to prepare a solution with a molar concentration of 2.35 mol / l, you need to pour 200 ml of HCL (36.3% wt.) In 800 ml of distilled water.
6. Consumption of hydrochloric acid to determine the carbonate content of rock samples.
The amount of concentrated acid spent on the study of the sample is calculated from the following reactions of interaction of carbonate substances, taking into account molecular weights(table 6) and molar concentration of acid (table 2):for calcite:
CaCO3 + 2HCL = CaCL2 + H2O + CO2
for dolomite:
CaMg(CO3)2 + 4HCL = CaCL2 + MgCL2 + 2H2O + 2CO2
for siderite:
FeCO3 + 2HCL = FeCL2 + H2O + CO2
The largest amount of acid is spent on the decomposition of dolomite, because. 1 g of CaMg(CO3)2 contains 21.691 mg-eq., 1 g of CaCO3 - 19.982 mg-eq., and 1 g of FeCO3 - 17.262 mg-eq. For the complete decomposition of carbonates, it is necessary to spend the same amount of meq. HCL.
1 ml of concentrated hydrochloric acid (35…38% wt.) contains 11.267…12.381 mEq. (Table 1). Therefore, the decomposition of 1 g of dolomite theoretically requires from 21.691 / 12.381 = 1.75 ml to 21.691 / 11.267 = 1.92 ml of concentrated acid (Table 7).
When conducting studies of rock samples, the consumption of concentrated acid should be at least 2 ml per 1 g of carbonate substances. An excess of acid is necessary for the normal course of a chemical reaction.
The calculated values of the volume of acid solutions required for the interaction of 1 g of carbonates with acid are given in table 8.
The consumption of aqueous solutions containing the optimal excess of hydrochloric acid for the complete decomposition of 1 g of carbonate rocks is shown in Table 9.
The actual volume of the acid solution used for testing one sample is determined by the manufacturer of the carbonatometers.
For carbonate meters of the KM series manufactured by NPP Geosfera LLC, the consumption of concentrated hydrochloric acid per sample is no more than 2.35 ml.
7. Sample preparation
To determine the carbonate content of a rock, it is necessary to weigh a crushed sample weighing from 500 mg to 1000 mg. A larger weight allows more reliable determination of the content of calcite and dolomite, especially in low-carbonate samples.
To obtain a sample weighing 1000 mg, it is necessary to select and grind at least 3 g of dry core flags or washed and dried particles of the base rock sludge.
After grinding the sample, it is necessary to sift the powder through a sieve with a mesh size of 0.056 mm or 0.063 mm.
If the sample is taken from an oil-saturated core or sludge, then after grinding, the sample should be extracted with an organic solvent (carbon tetrachloride CCl4 or chloroform CHCl3).
For extraction, the sifted powder must be poured heaped onto a piece of filter paper and, using a pipette, apply 30 ... 40 drops of solvent to it under the hood. After evaporation of the solvent from the sample, a sample must be taken for weighing.
Weighing should be carried out on electronic scales of at least accuracy class 3, with a readout resolution of at least 1 mg. It is recommended to pour the sample to be weighed on a substrate made of thick coated paper (for the convenience of subsequent filling into the container of the reaction chamber of the carbonate meter).
It should be taken into account that inaccurate weighing of the sample increases the error in determining carbonate content. For example, with a weighing error of ± 10 mg, the additional error in determining the carbonate content of a sample weighing 500 mg is ± 2%.
8. Neutralization of hydrochloric acid residues
After the completion of the reaction of interaction of carbonate substances with acid, a certain amount of HCl remains in the solution, depending on the carbonate content of the studied rock sample.When the content of carbonates in the sample is 100% wt. this amount corresponds to the excess volume of HCl introduced into the solution in excess of the calculated amount of acid required for the decomposition of 1 g of carbonate substances (table 7.8). If the carbonate content of the sample is less than 100% by weight, the excess HCl in the solution is increased by the amount of unreacted acid.
To neutralize HCl residues, an equal amount of mEq must be added to the solution. one of the substances that interact with hydrochloric acid (for example, sodium bicarbonate NaHCO3, potassium bicarbonate KHCO3, sodium carbonate Na2CO3, potassium carbonate K2CO3, sodium hydroxide NaOH or potassium hydroxide KOH).
The estimated amount of anhydrous substances used to neutralize the acid contained in 1 ml of aqueous HCl aqueous solutions of various concentrations is shown in table 10.
The amount of substance used to neutralize HCl residues after examining a 1 g rock sample can be determined based on the volume of the acid solution not consumed in the reaction.
Example.
When examining a rock sample weighing 1 g containing 85% calcite, 15 ml of an aqueous solution of HCl (1:6) prepared from an acid with a concentration of 38% wt. It is necessary to determine the amount of NaHCO3 to neutralize residual HCl after the reaction.
The calculated volume of acid solution for the decomposition of 1 g of CaCO3 is 11.3 ml (Table 8).
The excess HCl solution is 15.0 - 11.3 = 3.7 ml.
The calculated amount of unreacted acid is 11.3 (1 - 85/100) = 1.7 ml. Therefore, it is necessary to neutralize the acid in a solution with a volume 3.7 + 1.7 = 5.4 ml.
Hydrochloric (hydrochloric) acid - very strong, dangerous Chemical substance, which has a fairly wide application in many areas of human life.
Brine is hydrogen chloride (HCL, odorless thermal gas) combined with water (H2O). The boiling point depends on the concentration of the solution. The substance is flammable, storage condition: only in dry rooms.
Used in medicine, in the field of dentistry, for teeth whitening. If the stomach secretes an insufficient amount of juice (enzyme), hydrochloric acid solution is used as an adjuvant. In chemical laboratories, chlorine is a popular reagent for biochemistry experiments, sanitary standards and diagnostics.
Hydrochloric acid has become widely known in industry: dyeing fabrics, leather, soldering metal, removing scale, oxides, it is included in the manufacture of pharmaceuticals, as an oxidizing agent, and so on.
Properties of the chemical spectrum
Acid interacts with many metals, salts. It is considered quite strong and is on a par with chamois. The main reaction manifests itself in all groups of metals located to the left of hydrogen (magnesium, iron, zinc - electrical potentials).
As a result of such an impact, the formation of salts is obtained with the release of H into the air.
Hydrogen chloride solution in dilute form reacts with salts, but only with those that are formed less strong acids. Known to all sodium and calcium carbonate, after interaction with it, they decompose into water and carbon monoxide.
Nitric acid - a qualitative reaction to saline solution. To obtain it, it is necessary to add silver nitrate to this reagent, as a result, a white precipitate will form, from which a nitrogen substance is obtained.
With the help of this mixture of water and hydrogen, many interesting experiments are carried out. For example, dilute it with ammonia. As a result, you will get white smoke, thick, having the consistency of small crystals. Methylamine, aniline, manganese dioxide, potassium carbonate are reagents that are also affected by acid.
How hydrochloric acid is produced in the laboratory

The production of the substance is large-scale, the sale is free. In the conditions of laboratory experiments, a solution is produced by the action of high concentration sulfuric acid on ordinary kitchen salt (sodium chloride).
There are 2 methods for dissolving hydrogen chloride in water:
- Hydrogen is burned in chlorine (synthetic).
- Associated (off-gas). Its essence is in carrying out organic chlorination, dehydrochlorination.
The chemical properties of hydrochloric acid are quite high.
The substance lends itself well to synthesis during the pyrolysis of waste from organochlorine. This happens as a result of the breakdown of hydrocarbons with a complete lack of oxygen. You can also use metal chlorides, which are the raw materials of inorganic substances. If there is no concentrated sulfuric acid (electrolyte), take diluted.
Potassium permanganate is another way to get a salt solution.
With regard to the extraction of the reagent in natural conditions, then most often this chemical mixture can be found in the waters of volcanic waste. Hydrogen chloride is a component of the minerals sylvin (potassium chloride, looks like bones for games), bischofite. All these are methods to extract the substance in industry.
In humans, this enzyme is found in the stomach. A solution can be either an acid or a base. One of the most common methods of extraction is called sulfate.
How and why is it used

Perhaps, this is rightfully one of the important substances that is found and necessary in almost all branches of human life.
Application area localization:
- Metallurgy. Surface cleaning from oxidized areas, rust dissolution, pre-soldering treatment, tinning. Hydrochloric acid helps to extract small inclusions of metals from ores. Zirconium and titanium are obtained using the method of converting oxides into chlorides.
- Industry food technology. A low concentration solution is used as a food additive. Gelatin, fructose for diabetics contain a pure emulsifier. Ordinary soda also has a high content of this substance. On the packaging of goods you will see it under the name E507.
- The field of medicine. With an insufficient indicator of the acidic environment in the stomach and problems with the intestines. Low Ph leads to cancer. Even with proper nutrition, vitamins in abundance, the danger does not disappear, it is necessary to carry out tests to obtain juice from the gastric tract, because in an insufficiently acidic environment, useful substances are practically not absorbed, digestion is disturbed.
- Salt solution is used as an inhibitor - protection against dirt and infections, antiseptic action. For the manufacture of adhesive mixtures, ceramic products. It flushes heat exchangers.
- The procedure for purifying drinking water is also not complete without the participation of chlorine.
- Production of rubber, bleaching of fabric bases.
- You can care for your lenses with this solution.
- Mouthwash at home
- The substance is an excellent conductor of electricity.
Instructions for use

Hydrochloric acid can be used internally in medicine only as directed by a doctor. You cannot self-medicate.
The instruction is simple: the usual way to prepare a solution as a preparation is to stir until it completely disappears in water before use. 15 drops of medicine are prescribed for half a 200 gram glass. Take only during meals, 4 times a day.
Do not overdo it, this is not a panacea for diseases, it is important to consult a specialist. In case of an overdose, ulcerative formations occur on the mucous membrane of the esophagus.
Side effects and contraindications

Refrain from taking if you have a disposition to allergic reactions, this can adversely affect general functions organism.
Severe poisoning and burns

In case of contact with the skin of the product in a concentrated form, you can get a severe toxicological burn. The penetration of excess steam into the respiratory tract (larynx, throat) contributes to the induction of poisoning.
There is a strong choking cough, sputum may be with blood. Vision becomes cloudy, I want to constantly rub my eyes, mucous membranes are irritated. The iris does not respond to bright light.
Burning with hydrochloric acid is not as scary as sulfuric, but the vapors that can enter the digestive tract can lead to serious consequences of alkali intoxication.
The first sign (symptom) is the presence of elevated body temperature. Characteristic features of the action of this substance on the esophagus is visible in the following: wheezing in the lungs, vomiting, physical weakness, inability to take a deep breath, swelling of the airways.
When a large amount is ingested, the picture of toxicology is terrible: the volume of vomit increases, cyanosis of the face and arrhythmia are formed. The chest is compressed (asphyxia), followed by swelling of the larynx and death from pain shock.
With these symptoms, there is a certain classification of first aid actions.
It is very important to distinguish the stages of intoxication:
- If a person has been poisoned by vapors, it is urgent to take him out to clean air. Wash the throat with a solution of sodium bicarbonate, apply a compress to the eyes. Get to the hospital immediately.
- If the action of the acid is directed to the skin of a child or an adult, it is important to properly treat the burnt area. Rinse the skin for 15 minutes, and apply a burn ointment.
- If the solution harms the internal organs, an urgent cleansing of the stomach by probing and hospitalization is necessary.
Hydrochloric acid analogues in preparations

Since the allowable rate of a substance is used in medicine, it is contained in such medicines:
- Magnesium Sulfate.
- Calcium chloride.
- Reamberin.
Remember that for human consumption, hydrogen chloride acid is used exclusively in diluted form.